Carbon Footprint of Bufomix (budesonide / formoterol) Easyhaler® reduced by 12.1% between 2019 and 2023
Insights | 13/08/2024
Prescribing information available here and Adverse Event Reporting information can be found at the bottom of the page.
Growing environmental concerns are driving a transition towards the use of propellant-free inhaler devices such as dry powder inhalers over pressurised metered-dose inhalers. A series of three successive life cycle assessments (LCAs) conducted on the Easyhaler® product portfolio shows an 11.2% reduction in the average carbon footprint (CF) between the years 2019 and 2023. Bufomix (budesonide/formoterol) Easyhaler, the only Easyhaler range available in the Republic of Ireland showed a 12.1% reduction in the average CF during that time. These findings demonstrate that a reduction in the climate impact of pharmaceutical products can be achieved without modifying the product itself.1
- The results of three successive life cycle assessments conducted on the Easyhaler dry powder inhaler product portfolio were analysed to evaluate the evolution in environmental impact and carbon footprint of Easyhaler products over time.
- The nonweighted average carbon footprint of the Easyhaler decreased by 11.2% between years 2019 to 2023. Individual product reductions varied from 5.0% to 6.8% between the assessments.
- The results demonstrate the effectiveness of sustainability measures within the value chain and highlight that a reduction in the climate impact of pharmaceutical products can be successfully achieved without modifying the product itself.
According to The National Health Service, inhalers significantly contribute to the environmental footprint of healthcare, representing 13% of the treatment-related CF.2 In response to growing environmental concerns, there is a shift towards favouring propellant-free inhaler devices over pressurised metered-dose inhalers, due to notable differences in their CF.1
This study1 addressed the results of three LCAs conducted on the Easyhaler product portfolio in 2019, 2021, and 2023. These cradle-to-grave LCAs evaluated the changes in environmental impact of the product over time, considering their entire life cycle from raw material extraction to disposal. The LCAs assessed the CF and nine other environmental impact categories to ensure no burden shifting occurred.
The 2019 LCA covered four products of the Easyhaler product portfolio, while the two subsequent assessments included all six products. Bufomix (budesonide/formoterol) Easyhaler is the only easyhaler range available in the Republic of Ireland). In 2023, the LCA also included, for the first time, the assessment of the protective cover sometimes used with Easyhaler. The analyses of all three LCAs were conducted identically by independent third-party consultant, allowing the accurate analysis of changes in CF over time.
Between the assessments of 2019 and 2023, the nonweighted average CF of the Bufomix Easyhaler decreased by 12.1% (Table 1). In the LCA 2023, the CF of Bufomix Easyhaler was 452 grams of carbon dioxide equivalent (gCO2e), while the CF of the protective cover was 66 gCO2e. These emissions aligned with the lower end of the CF range for dry powder inhalers. The protective cover's climate impact was one-tenth of the product itself, and reusability further mitigates its environmental effects.
The largest contributors to the reduction in CF emissions included the manufacturing of inhaler components, the assembly of the final product, and the raw materials for inhaler components. No burden shifting was observed among environmental variables.
Table 1. Reduction of direct greenhouse gas emissions of Bufomix Easyhaler® from 2019 to 2023. Emissions of one inhaler in 2019, 2021 and 2023 (g CO2e) and relative change of emissions is presented. The 2023 data also includes gCO2e per actuation.
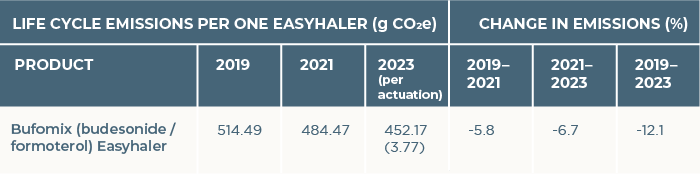
gCO2e, grams of carbon dioxide equivalent; NA, not available.
The consistent decrease in the average CF over the study period demonstrates the effectiveness of sustainability measures within the value chain and highlights that a reduction in the climate impact of pharmaceutical products can be achieved without modifying the product itself. Instead, it can be attained by reducing the environmental impact of manufacturing processes, distribution, and disposal. The LCA results aid in identifying stages in the product life cycle with the most significant environmental impacts, allowing for facilitation of targeted actions to those stages.
For further insights on Easyhaler’s reduced carbon footprint, see:
- Switching to the Easyhaler Device to control Asthma and COPD: A literature review (wehale.life)
- Climate (orion.fi)
References:
- Inget M, Hisinger-Mölkänen H, Howard M, Lähelmä S, Paronen N. Cradle-to-Grave Emission Reduction for Easyhaler Dry Powder Inhaler Product Portfolio. Pulm Ther. 2023 [published online ahead of print, 2023 Sep 25].
- Wilkinson A, Woodcock A. The environmental impact of inhalers for asthma: A green challenge and a golden opportunity. Br J Clin Pharmacol. 2021. https://doi.org/10.1111/bcp.15135.
Date of preparation: August 2024 / EASYH-4158
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |

