Clinical effectiveness of
Bufomix (Budesonide/Formoterol) Easyhaler® for patients with poorly controlled obstructive airway disease: a real-world study of patient-reported outcomes
Insights |
23/09/2022
Prescribing information available here and Adverse Event Reporting information can be found at the bottom of the page.
The clinical effectiveness and patient satisfaction of Bufomix (budesonide/formoterol) Easyhaler® was evaluated in patients with asthma, chronic obstructive pulmonary disease (COPD), and asthma-COPD overlap (ACO) in daily clinical practice.
Tamási et al. 2018. Adv Ther
- The clinical effectiveness and patient satisfaction of Bufomix (budesonide/formoterol) Easyhaler® was evaluated in patients with asthma, chronic obstructive pulmonary disease (COPD), and asthma-COPD overlap (ACO) in daily clinical practice.
- The use of Bufomix Easyhaler® significantly improved disease control, quality of life, and lung function in all patient groups (P≤0.002).
- Patients were satisfied with the use of Bufomix Easyhaler®, and most patients learned to use it in less than 5 minutes.
Inhalation is the most recommended route for administering medication for asthma and COPD patients. Therefore, inhalation technique and inhaler device are important factors in achieving an effective clinical response. Here, the effectiveness of Bufomix Easyhaler® was assessed in a 12-week real-world, multicenter, open-label, non-randomized, non-interventional study among 1498 patients with asthma, COPD, or ACO in Hungary. One-third (30.4%) of the patients were newly diagnosed and 69.6% were switched to Easyhaler Dry Powder Inhaler (DPI) from other inhalers, most commonly from metered dose inhaler (MDI) (24%), budesonide/formoterol originator Dry Powder Inhaler (DPI) (19%), or Salmeterol/fluticasone originator (DPI)
Clinical effectiveness in patients with asthma or ACO (N=720) was evaluated based on disease control (Asthma Control Test, ACT), quality of life (mini-Asthma Quality of Life Questionnaire, mini-AQLQ), and lung function (forced expiratory volume in one second, FEV1% predicted measurement). Similarly, in the COPD and ACO patient group (N=877), disease control was evaluated by COPD assessment test (CAT), quality of life based on the modified Medical Research Council (mMRC) dyspnea scale, and lung function by FEV1% predicted measurements. Satisfaction among patients who switched from other inhalers (N=1043) was recorded by a questionnaire in a scale from 1 (very good) to 6 (unsatisfying).
The results showed that the use of Bufomix Easyhaler significantly improved disease control as demonstrated by the changes in the ACT score (from 14.2 to 21.0, P<0.001) and CAT score (from 24.2 to 18.2, P<0.001) (Figure 1). Treatment with Bufomix Easyhaler also improved the quality of life in all patient groups (P<0.001). In addition, the use of Bufomix Easyhaler resulted in significant improvement in lung function across all patient groups (P<0.001), and importantly, also in patients who were switched from previous inhalers (P<0.001).
After 12 weeks of treatment with Bufomix Easyhaler, the majority (87.2%) of asthma patients were able to reduce the use of a reliever inhaler. More than 90% of physicians considered Bufomix Easyhaler as very easy or easy to teach and use, and most patients (73.8%) learned the technique in less than 5 minutes. Notably, 72.4% of the patients rated Bufomix Easyhaler as “very good”, whereas corresponding results for MDI, budesonide/formoterol originator (DPI) (19%) and Salmeterol/fluticasone originator (DPI) were 12.7%, 17.3%, and 18%, respectively.
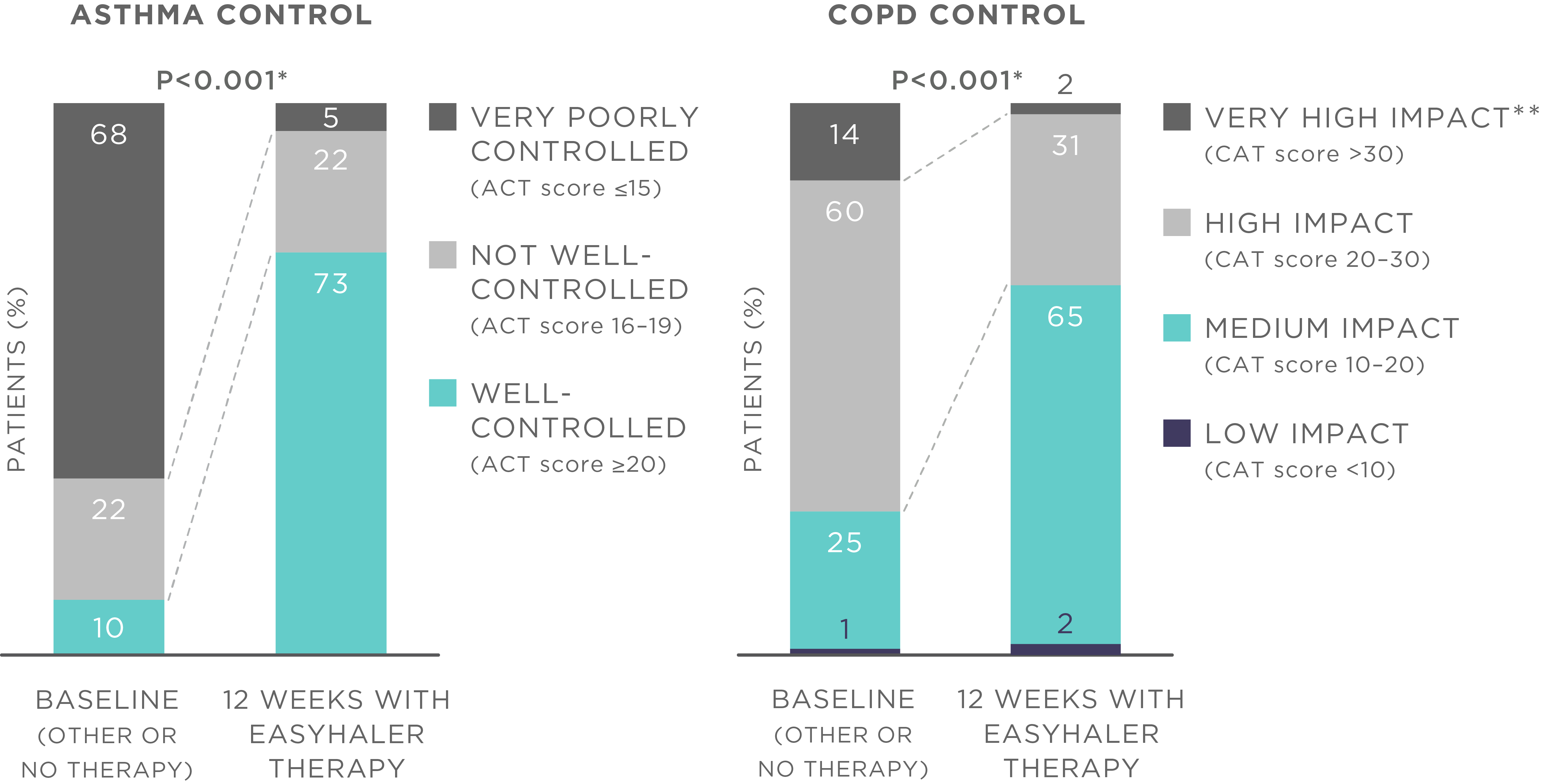
Figure 1. Bufomix Easyhaler therapy improves disease control among patients with asthma, COPD, and ACO. N (total) = 1498 patients (621 with asthma, 778 with COPD, and 99 with ACO; 455 newly diagnosed patients and 1043 patients switching from another inhaler device). *P<0.001 for average ACT and CAT score, **Impact of COPD symptoms on everyday life. Patients received 12 weeks of budesonide/formoterol Bufomix Easyhaler therapy.
This real-life study showed that Bufomix Easyhaler significantly improved disease control, quality of life and lung function among patients with asthma, COPD, and ACO (all P ≤ 0.002). The inhaler was considered easy to teach and use, and the majority of patients rated the user satisfaction of Bufomix Easyhaler with the highest grade.
Reference:
Tamási L, Szilasi M, Gálffy G. Clinical Effectiveness of Budesonide/Formoterol Fumarate Easyhaler® for Patients with Poorly Controlled Obstructive Airway Disease: a Real-World Study of Patient-Reported Outcomes. Adv Ther. 2018 Aug;35(8):1140-1152.
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |
Date of preparation: July 2024 / EASYH-2634(1)

