Comprehensive asthma care with Bufomix (budesonide/formoterol) Easyhaler® maintenance and reliever therapy (MART)
Insights | 20/08/2020Prescribing information available here
- Global Initiative for Asthma (GINA) guidelines recommend maintenance and reliever therapy (MART) with a combination of an inhaled corticosteroid (ICS) and a long-acting β2-agonist formoterol for the treatment of asthmatic patients with moderate or moderate-to-severe disease.
- ICS-formoterol MART reduces asthma exacerbations and maintains disease control at lower corticosteroid load compared to conventional treatment approaches.
- Bufomix (budesonide/formoterol) Easyhaler® provides both maintenance and reliever therapy for asthmatic adults and adolescents within a single inhaler.
- 80/4.5 and 160/4.5mcg are the strengths indicated for MART therapy
The use of ICS-formoterol combination in a single inhaler offers an enhanced treatment approach for asthmatic patients in need of MART. According to GINA guidelines, low dose ICS-formoterol MART is the preferred controller and reliever option for adult and adolescent (≥12 years) patients with moderate or moderate-to-severe asthma (Figure 1).
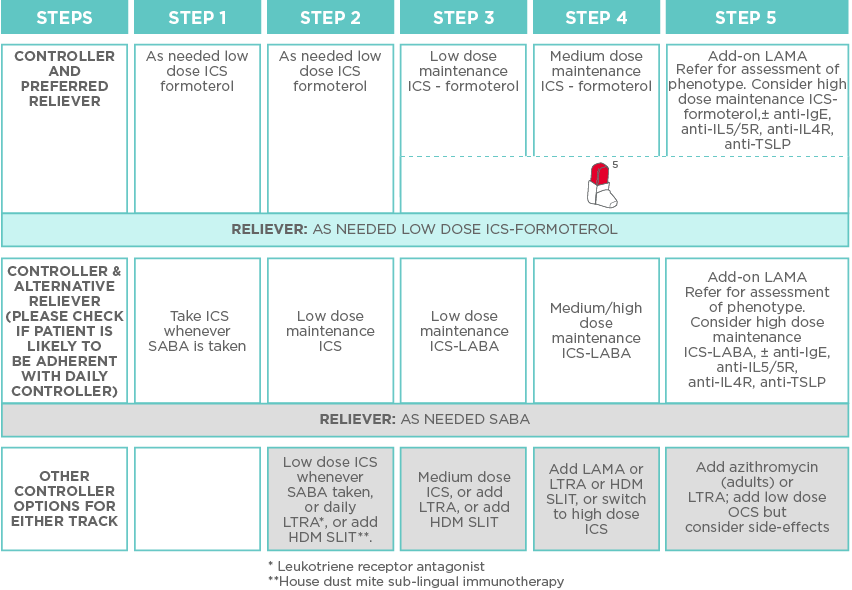
Figure 1. A stepwise treatment approach for asthmatic adults and adolescents (≥12 years) according to GINA. The GINA guidelines recommend low dose ICS-formoterol MART as the preferred controller and reliever option for patients with moderate or moderate-to-severe asthma.1 Bufomix Easyhaler® provides MART within a single inhaler.2
The GINA report describes several clinical studies that have indicated the benefits of ICS-formoterol maintenance and reliever therapy when compared with conventional treatment regimens among patients with moderate or moderate-to-severe disease. For instance, low dose ICS-formoterol MART reduces asthma exacerbations compared to traditional maintenance therapy with SABA as reliever.1 Furthermore, patients treated with budesonide-formoterol MART maintain similar asthma control at lower corticosteroid load compared to fixed-dose treatments3, and also experience significantly less asthma exacerbations than those receiving fixed-dose therapy with as-needed terbutaline3,4 or formoterol4 in separate devices.
Bufomix (budesonide-formoterol) Easyhaler is a combination therapy option for asthma patients in need of single-inhaler MART. Bufomix Easyhaler is indicated for both maintenance and reliever therapy in asthmatic adults and adolescents (≥12 years old) and is available in two different dose strengths: 80/4.5 mcg/dose and 160/4.5 mcg/dose. Bufomix Easyhaler MART therapy is especially worth considering for patients with inadequate asthma control and in frequent need of reliever medication, as well as for patients with asthma exacerbations in the past requiring medical intervention.2
The evidence from in vitro pharmacokinetic assessments, together with clinical efficacy and safety studies, demonstrates therapeutic equivalence between different strengths of Bufomix Easyhaler and the reference originator product.5–7 Besides therapeutic efficacy, Bufomix Easyhaler has been shown to provide more accurate and consistent dose delivery than the reference inhaler, even with low patient inhalation flow rates.8 In a recent real-world study, switching patients from budesonide-formoterol originator product to an equivalent dose Bufomix Easyhaler resulted in a significant improvement in asthma control and health-related quality of life (ACT and mini-AQLQ, P<0.001).9
Altogether, the clinical studies and asthma management guidelines suggest that maintenance and reliever therapy with a combination of an inhaled corticosteroid and a long-acting ß2-agonist provides comprehensive asthma care, and may even improve asthma outcomes with a lower drug load.1.3.4
For further insight into the budesonide-formoterol Easyhaler® studies, see:
Switching to budesonide-formoterol Easyhaler® improves disease control and health-related quality of life
Clinical effectiveness of budesonide-formoterol fumarate Easyhaler® for patients with poorly controlled obstructive airway disease: a real-world study of patient-reported outcomes
Budesonide-formoterol Easyhaler®: performance under simulated real-life conditions
Evaluation of the efficiency of single-inhaler combination therapy with budesonide-formoterol fumarate in patients with bronchial asthma in daily clinical practice
Switching asthma and COPD patients to budesonide-formoterol Easyhaler® leads to improved clinical outcomes and quality of life in real-world clinical practice
The availability of Easyhaler products varies between countries. Bufomix (budesonide-formoterol) Easyhaler is the only preparation available in the Republic of Ireland.
Prescribing information available here
References:
- Global Initiative for Asthma (GINA), Global Strategy for asthma management and prevention, 2022 Main report available at: https://ginasthma.org/gina-reports/ (Accessed July 2022)
- Budesonide-formoterol Easyhaler® 80/4.5 and 160/4.5 mcg/dose. SmPC. Orion Pharma.
- Kuna P, Peters MJ, Manjra AI, Jorup C, Naya IP, Martínez-Jimenez NE, Buhl R. Effect of budesonide/formoterol maintenance and reliever therapy on asthma exacerbations. Int J Clin Pract 2007;61(5):725–36.
- Rabe K, Atienza T, Magyar P, Larsson P, Jorup C, Lalloo U. Effect of budesonide in combination with formoterol for reliever therapy in asthma exacerbations: a randomized controlled, double-blind study. Lancet 2006;368(9537):744–53.
- Lähelmä S, Vahteristo M, Metev H, Taseva M, Stamatova N, Bartha A, Schlezák J, Sairanen U. Equivalent bronchodilation with budesonide/formoterol combination via Easyhaler and Turbuhaler in patients with asthma. Respir Med 2016;120:31–35.
- Lähelmä S, Sairanen U, Haikarainen J, Korhonen J, Vahteristo M, Fuhr R, Kirjavainen M. Equivalent lung dose and systemic exposure of budesonide/formoterol combination via Easyhaler and Turbuhaler. J Aerosol Med Pulm Drug Deliv 2015;28(6):462–73.
- Malmberg LP, Everard ML, Haikarainen J, Lähelmä S. Evaluation of in vitro and in vivo flow rate dependency of budesonide/formoterol Easyhaler®. J Aerosol Med Pulm Drug Deliv 2014;27(5):329–40.
- Haikarainen J, Selroos O, Löytänä T, Metsärinne S, Happonen A, Rytila P. Budesonide/Formoterol Easyhaler®: Performance under simulated real-life conditions. Pulm Ther 2017;3:125–38.
- Syk J, Vinge I, Sörberg M, Vahteristo M, Rytilä P. A multicenter, observational, prospective study of the effectiveness of switching from budesonide/formoterol Turbuhaler® to budesonide/formoterol Easyhaler®. Adv Ther 2019;36(7):1756–69.
Date of preparation: August 2022 / EASYH-887(1)

