Easy, convenient, and environmentally friendly asthma and COPD care with Easyhaler®
Insights | 25/08/2020Prescribing information available here and Adverse Event Reporting information can be found at the bottom of the page.
- Inhalers should deliver an accurate and consistent pulmonary dosing during repeated use.
- An ideal inhaler should be: effective, reproducible, precise, stable, comfortable, versatile, and environmentally friendly.1
- Dry powder inhalers (DPIs) have benefits compared to pressurised metered-dose inhalers (pMDIs), as they are breath-activated, easy and convenient to use, and more environmentally friendly.1
- The Easyhaler® DPI has many features that make it close to an ‘ideal’ inhaler.
Drugs used by patients with asthma or COPD are delivered by inhalers such as pMDIs and DPIs. Inhaler devices should consistently deliver an expected, reproducible and breathable-sized drug dose. To fulfil the concept of an ‘ideal’ inhaler, the device should be: 1) effective (i.e. able to guarantee inhalation of an adequate fraction of drug, in breathable-size particles, independently, as much as possible, of changes in patient inspiratory flow); (2) reproducible (i.e. able to allow inhalation of the same drug amount, also in terms of its breathable fraction); (3) precise (i.e. able to consent to know, at any moment, the number of doses in the device, and whether inhalation was correctly performed); (4) stable (i.e. able to protect the drug(s) from the effects of temperature and/or humidity variation); (5) comfortable (i.e. easy to use in different circumstances and including several doses of the drug(s) for long-term use); (6) versatile (i.e. permit the administration of different drugs); (7) minimise impact on the environment.1
pMDIs utilise a propellant to generate a metered dose of an aerosol, whereas DPIs are run by the patient’s inspiratory flow. Both DPIs and pMDIs can provide an equivalent effectiveness if they are used correctly. However, patients often experience problems using pMDIs due to poor coordination between dose actuation and inhalation. The advantage of DPIs over pMDIs include breath-activation, which overrides the need to coordinate dose actuation with inhalation. Currently no available inhaler can be considered ‘ideal’. Nevertheless, some devices closely resemble the concept of the best possible inhaler device in a real-world setting.1
Easyhaler® is an environmentally friendly multidose reservoir-type DPI. Several studies have demonstrated the advantages of Easyhaler over other multi-dose DPI devices, and in real-world conditions.1,2,3
In vitro studies have shown that Easyhaler performs well by delivering drug doses accurately and consistently under simulated real-life conditions and irrespective of the patient inhalation flow rate (Figure 1).2-4 Easyhaler’s set of inhalation characteristics for formulation, deaggregation, and delivery of the emitted dose ensures that the vast majority of children, adolescents, adults, and elderly patients with asthma or COPD are capable of achieving the PIF level of 30 L/min or higher required for optimal Easyhaler use.5–8 The proper use of an inhaler correlates directly with clinical effectiveness as it improves the drug delivery, efficacy, and, finally, disease control.
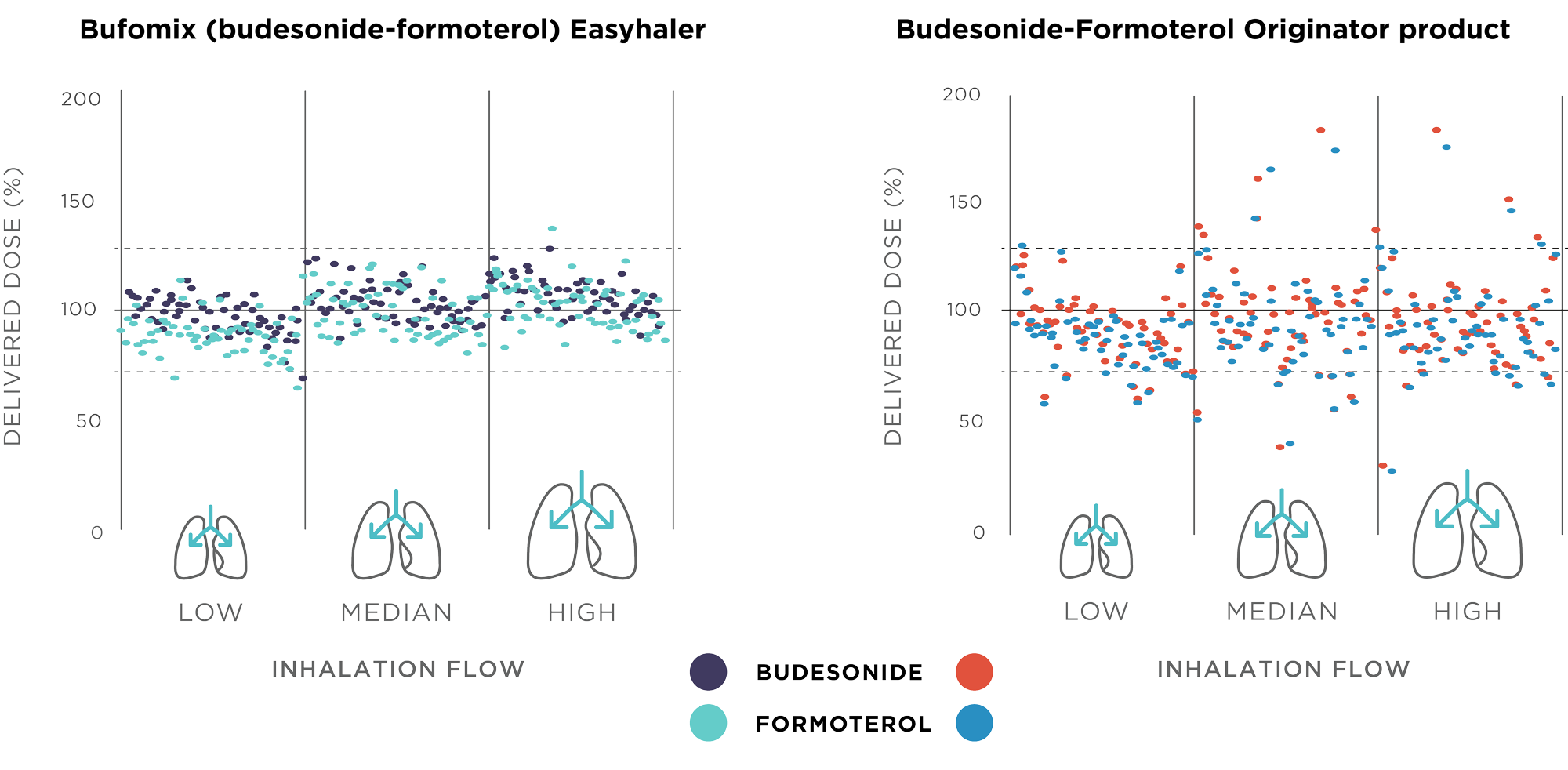
Figure 1. Dose delivery of budesonide and formoterol fumarate (strength 160/4.5 mcg) from two budesonide-formoterol multi-dose DPIs, Bufomix Easyhaler® and originator product, at three different inhalation flow rates. Results originally published in Haikarainen et al. 2017.2
Most patients value the comfort aspects of Easyhaler: it is portable, and easy to learn, to use and keep clean during daily activities. Moreover, reports show that asthma and COPD patients of all ages are highly satisfied with Easyhaler.3 A recent retrospective study also shows that patients may successfully switch to Easyhaler therapy from other inhalers with no reduction in clinical effectiveness or increased cost.10 Furthermore, it has been demonstrated that after 3 months from switching to Easyhaler from previous inhalers, most patients were able to achieve improved disease control.10
Overall, the study findings suggest that in real-world conditions Easyhaler fulfills many of the criteria of an ideal inhaler.2,3
Bufomix (Budesonide/Formoterol) Easyhaler Contraindications and Undesirable Effects
Contraindications:
Hypersensitivity to the active substances or lactose monohydrate (which contains small amounts of milk protein).
Undesirable effects:
Common (≥1/100 to < 1/10): Candida infections in the oropharynx, pneumonia (in COPD patients) headache, tremor, palpitations, mild irritation in the throat, coughing, dysphonia including hoarseness
For further insight into the Easyhaler® studies, see:
Budesonide-formoterol Easyhaler®: performance under simulated real-life conditions.
References:
As reviewed in:
| 1. | Lavorini F. Easyhaler®: an overview of an inhaler device for day-to-day use in patients with asthma and chronic obstructive pulmonary disease. Drugs Context 2019;8:212596. |
Original articles:
Date of preparation: July 2024 / EASYH-907(2)
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |

