Evaluation of the efficiency of single-inhaler combination therapy with budesonide/formoterol fumarate in patients with bronchial asthma in daily clinical practice
Insights | 18/01/2022Prescribing information available here and Adverse Event Reporting information can be found at the bottom of the page
- The clinical efficacy of Bufomix Easyhaler (budesonide/formoterol fumarate dihydrate) inhaler in daily practice was assessed in 2200 patients with bronchial asthma.
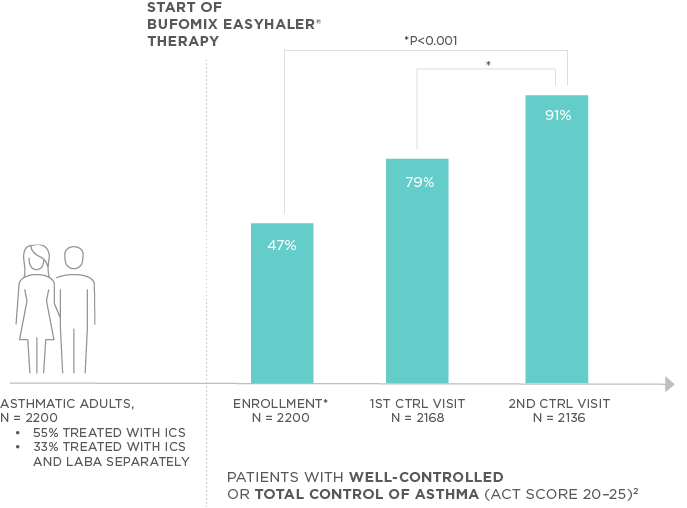
- The proportion of patients with well-controlled or totally controlled asthma, as defined by ACT score of ≥20, increased from 46.6% at enrollment to 90.8% at the second control visit (p<0.001).
- Treatment adherence rate was above 95% at the second control visit (p<0.001), and patients reported excellent satisfaction and ease of use of the inhaler.
The combination of the inhaled corticosteroid (ICS) budesonide and long-acting β2-agonist (LABA) formoterol has been proven effective in controlling bronchial asthma through suppression of chronic inflammation and reduction of airway hyperresponsiveness. In this real-life post-authorization efficacy study (PAES), Bufomix Easyhaler - a fixed dose budesonide/formoterol fumarate combination dry powder inhaler product– was shown to be highly effective and easy to use in daily clinical practice. Excellent medical adherence and patient satisfaction were also reported.
The clinical efficacy of Bufomix Easyhaler was assessed in a non-randomized, open-label, and non-interventional multicenter study in Poland. This real-life study was conducted in 2200 combination-naïve adult asthma patients who had recently (at least 14 days before enrollment) started budesonide/formoterol treatment (320/9 mcg or 160/4.5 mcg per inhalation). Asthma Control Test (ACT) was recorded on enrollment and during two control visits after intervals of 8–12-weeks; mean exposure to Bufomix Easyhaler was 142 ± 27 days. Prior to the start of treatment, over 50% of patients had been treated for asthma for longer than 5 years; 54.6% were using an ICS and 33.4% were using LABA and ICS therapies separately. Most patients had mild chronic (32.6%) or moderate persistent asthma (59.8%) (GINA 2015 classification). The percentage of patients with well-controlled or totally controlled (ACT score 20–25 points), and poorly controlled asthma (ACT score <15 points) was the primary endpoint of this study.
|
|
The proportion of patients with well-controlled or totally controlled asthma increased from 46.6% at the first visit (enrollment) to 90.8% at the second control visit (p<0.001) (Figure 1); from 41.1% to 87.7% (p<0.001) for the higher dose and 55.0% to 95.5% (p<0.001) for the lower dose. The proportion of patients with poorly controlled asthma decreased from 14.9% to 1.2% (p<0.001). Adherence to the use of Bufomix Easyhaler increased from 88.0% to 95.3% (MAQ, Medication Adherence Questionnaire; p<0.001). Patient satisfaction was over 90% at visit 3: the product was considered portable, easy to use and hygienic. |
This real-life study showed that Bufomix Easyhaler demonstrates good clinical efficacy among adult asthma patients in daily clinical practice, in outpatient care, as measured by ACT. Patients reported excellent satisfaction and ease of use of the inhaler.
Bufomix Easyhaler Contraindications and Undesirable effects
Contraindications:
Hypersensitivity to the active substances or lactose monohydrate (which contains small amounts of milk protein).
Undesirable Effects:
Common (≥1/100 to < 1/10): Candida infections in the oropharynx, pneumonia (in COPD patients) headache, tremor, palpitations, mild irritation in the throat, coughing, dysphonia including hoarseness.
See SmPC for full list of adverse reactions
Date of preparation: December 2025 / EASYH-1958(2)
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |