Global Initiative for Asthma — What is new in GINA 2022?
Insights | 07/09/2022
- The 2022 updated version of the Global Strategy for Asthma Management and Prevention has been published, including minor additions to the GINA treatment figures.1
- New biologic therapy options have been added, and the use of oral corticosteroids for maintenance therapy is now stated as a last resort option.
- Several other recommendations have been reviewed and updated, including information on COVID-19 and asthma.
The Global Strategy for Asthma Management and Prevention is a report written by an international panel of experts on the Global Initiative for Asthma (GINA) Scientific Committee. This summary outlines the key updates made in the yearly review in 2022.1
Updated guidance on COVID-19 and asthma
Further evidence confirms that there is no increased risk of severe COVID-19 infection in patients with well-controlled mild to moderate asthma, but the risk is increased in patients who have recently needed oral corticosteroids and in hospitalized patients with severe asthma. GINA recommends that all patients with asthma should be up to date with the COVID-19 vaccine, including booster shots if available. Advice on infection control practices for aerosol-generating procedures has also been updated.
Diagnosis and management of asthma
The diagnostic approach flowchart has been supplemented with guidance on diagnostic steps in patients already using controller treatment. Besides that, guidance on asthma diagnostics in low to middle income countries has been added. This is important, as the majority of asthma-related morbidity and mortality is experienced in these countries.
GINA has updated the guidance on reliever use frequency as a marker for symptom control. Historically, the use of short-acting β2-agonists (SABA) more than two days a week was considered as a sign of a need for a controller medication. However, the use of ICS-formoterol reliever more than two days a week should not be used in the assessment of symptom control. Instead, the average frequency of ICS-formoterol over the past four weeks should be considered when reviewing the maintenance controller dose.
Changes in the GINA treatment figures
The GINA treatment figure for adults and adolescents with two optional treatment tracks has undergone only minor additions. The preference for the track with ICS-formoterol as reliever is further reinforced. Anti-thymic stromal lymphopoietin (TSLP) has been added to treatment step 5 as a new biologic therapy option. The other controller options section has been clarified regarding their indications and limitations, in the treatment figure for adults and adolescents and the one for children aged 6–11 years. In the treatment figure for children ≤5 years, a short course ICS has been included as an option for patients with intermittent viral wheezing. GINA also now states that for maintenance therapy, oral corticosteroids should be the last resort for all age groups.
The GINA guide and decision tree for difficult-to-treat and severe asthma in adults and adolescents have been revised. New information on the use of anti-TSLP, anti-interleukin-4 receptor (IL4R) and anti-immunoglobulin E (IgE) therapies has been added, as well as new guidelines for patients with elevated blood eosinophils. These changes together with other key changes in the 2022 GINA report are described in Table 1.
Table 1. Other additions and changes in the GINA report 2022.
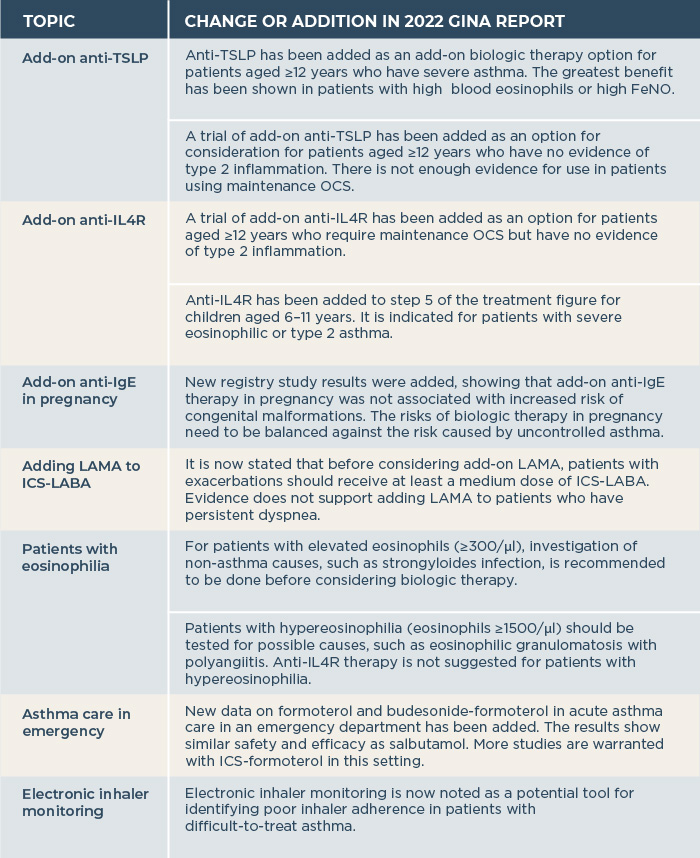
ICS, inhaled corticosteroids; IgE, immunoglobulin E; IL4R, interleukin-4-receptor; LABA, long-acting β2-agonists; LAMA, long-acting muscarinic antagonists; OCS, oral corticosteroids TSLP, thymic stromal lymphopoietin.
Other key changes
The definition of severe asthma is based on difficulty to treat, whereas the corresponding definition for mild asthma is much less clear. GINA recommends avoiding the use of the term ‘mild asthma’ in clinical practice, since it may mislead to beliefs that the asthma does not contain any risks or that no controller would be needed.
GINA continues to recommend written asthma action plans, but the term ‘written’ is now clarified to include printed, digital, or pictorial plans. Patients should be given documented instructions about how to change medications and when to seek medical advice if their asthma worsens, instead of only verbally communicated advice.
Local and national guidelines as well as licensed drug doses and indications should be considered when assessing and treating patients.
References:
- Global Initiative for Asthma (GINA). Global Strategy for Asthma Management and Prevention (2022 update). Available at https://ginasthma.org/gina-reports/. Referenced 2022-05-10.
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |
Date of preparation: September 2022 / EASYH-2642



