Global strategy for the diagnosis, management, and prevention of COPD - What has changed in GOLD 2023?
Insights | 13/09/2023
- The GOLD annual report reviews the latest research publications to improve the prevention and treatment of COPD.
- The ABCD Assessment Tool has been revised and changed to the new ABE Assessment Tool, and the use of a long-acting muscarinic antagonist together with long-acting β2-agonist is recommended as the initial treatment.
- Several other recommendations have been revised and updated, including definitions of COPD, exacerbation, and role of ICS therapy in COPD.
Changes in the GOLD Assessment Tool and pharmacotherapy of COPD
The guidelines for initial assessment and initiation of pharmacological management of COPD have been updated. The previous ABCD Patient Assessment Tool has been revised and changed to the ABE Assessment Tool. Groups A and B are unchanged, but groups C and D have been merged into a single group E to recognize the clinical relevance of exacerbations, independent of the level of symptoms. The new ABE Assessment Tool and characteristics of patient groups are shown in Figure 1.
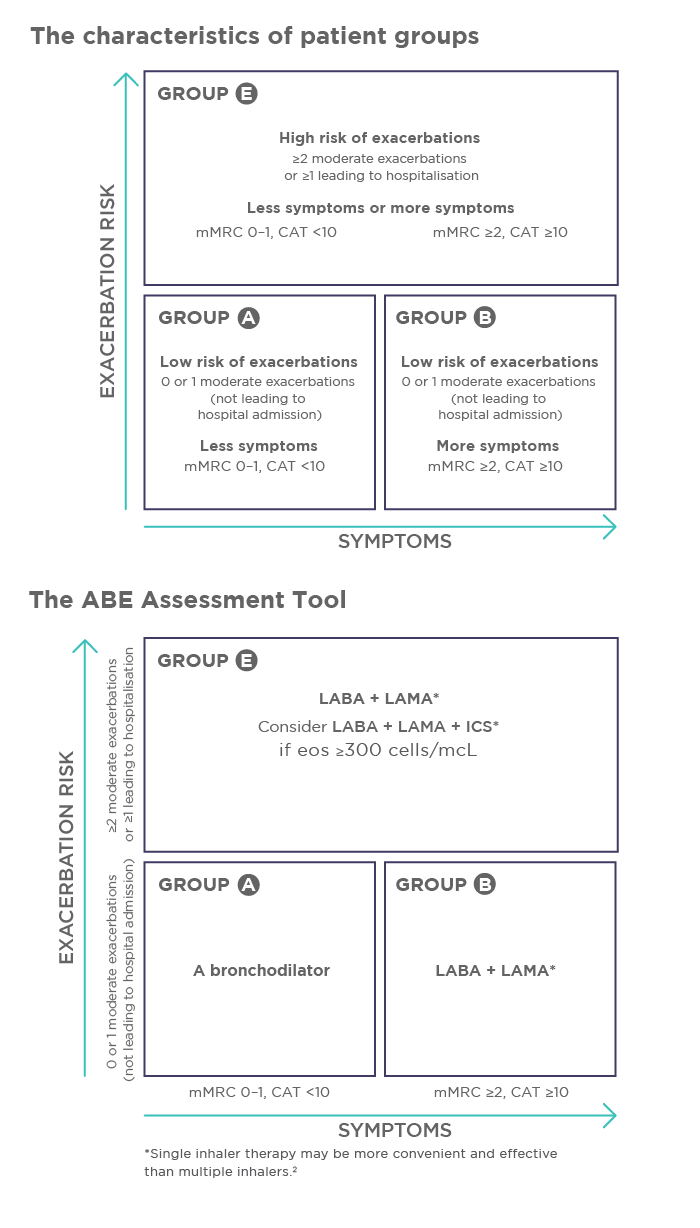
Figure 1. The ABE Assessment Tool for initial pharmacological treatment and the characteristics of patient groups. CAT, COPD Assessment Test; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, modified Medical Research Council dyspnea questionnaire.
GOLD has updated the recommendation for pharmacological management of stable COPD. Firstly, the use of a long-acting muscarinic antagonist (LAMA) together with a long-acting β2-agonist (LABA) is the preferred choice when initiating COPD treatment. Secondly, adding an inhaled corticosteroid (ICS) to COPD treatment is recommended for patients satisfying any of the following conditions: blood eosinophil count is ≥300 cells/microlitre, history of hospitalisation(s) for exacerbations, ≥2 moderate exacerbations per year, or concomitant asthma. The triple combination therapy, LABA-LAMA-ICS, can be given as single or multiple inhaler therapy.
Updates on definition of COPD and exacerbation
One of the key changes in the 2023 GOLD report include a revised definition of COPD. The revised definition of COPD now refers to complexity of the underlying disease without mentioning risk factors.
“COPD is a heterogeneous lung condition characterized by chronic respiratory symptoms (dyspnea, cough, sputum production) due to abnormalities of the airways (bronchitis, bronchiolitis) and/or alveoli (emphysema) that cause persistent, often progressive, airflow obstruction.”
Another apparent change in the 2023 report is a completely new definition of COPD exacerbation. The previous definition was unclear and did not include a link between a time window and symptoms. The new definition is as follows:
“…an event characterized by increased dyspnea and/or cough and sputum that worsens in <14 days which may be accompanied by tachypnea and/or tachycardia and is often associated with increased local and systemic inflammation caused by infection, pollution, or other insult to the airways.”
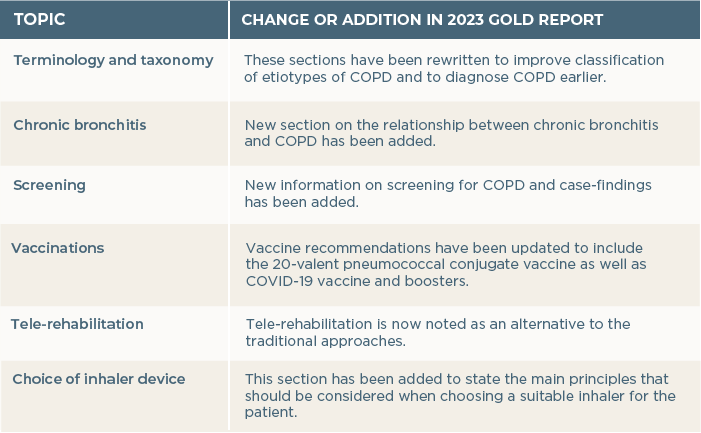
Other key changes and additions in the 2023 GOLD report are described in Table 1.
Table 1. Other changes or additions in the 2023 GOLD report.
The full GOLD report is available at the GOLD website.
For further insights on disease prevention and treatment, see:
Managing COPD correctly leads to less exacerbations and better quality of life (wehale.life)
References:
- Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD). GOLD 2023 Key changes summary. Available at https://goldcopd.org/2023-gold-report-2/.
- Global Initiative for Chronic Obstructive Pulmonary Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. Available at https://goldcopd.org/2023-gold-report-2/.
Date of preparation: September 2023 / EASYH-1957(1)
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |




