Inhaler technical features and patient education in supporting successful treatment of asthma and COPD
Insights | 11/08/2020Prescribing information available here and Adverse event reporting information can be found at the bottom of this page.
- Careful selection of an inhaler based on the patient's needs and educating them in the correct inhalation technique are key steps in successful inhalation therapy of asthma and COPD.
- Easyhaler ensures accurate and consistent dosing with variable inhalation flows and even patients with compromised inspiratory effort are able to reach the optimal peak inspiratory flow (PIF) rate of 30 L/min.1,2
- Real world studies on Easyhaler suggest that the majority of patients are satisfied with Easyhaler and consider the inhaler to be easily portable as well as easy to learn and use in everyday life.1
The main goals of pharmacological therapy of asthma and COPD are to control symptoms, reduce the frequency and severity of exacerbations, improve exercise tolerance and health status, and reduce mortality.1 Achieving optimal control of these chronic diseases requires careful selection of both the medication and inhaler device which best serve the needs of the patient. It is also important that the patient is an active participant in the decision-making process. Studies suggest that errors in inhaler use are common and may greatly affect the efficacy of the therapy. Furthermore, patient satisfaction with an inhaler is associated with treatment adherence and disease control.2
Easyhaler® devices are breath-actuated dry powder inhalers (DPIs) precisely designed to ensure efficient and consistent delivery of the drug to the site of action. The patient’s inhalation energy, together with the internal characteristics of the inhaler, generates a turbulent airflow which disaggregates the drug particles and delivers them to the airways. Easyhalers are high or medium/high internal resistance inhalers, which makes them less dependent on high PIF.1 Studies addressing the inspiratory profiles of asthma and COPD patients suggest that the Easyhaler PIF requirement of 30 L/min doesn’t usually limit the use of the inhaler, and is sufficient to ensure accurate and consistent dosing across different patient groups.1,2 Easyhaler also provides consistent dosing through varying inhalation flows (Figure 1).1,2 Furthermore, Easyhaler has been demonstrated to perform well when exposed to different environmental conditions such as high temperature and humidity variation as well as after vibration and dropping.1
The most common mistakes in the use of DPIs are exhaling into the inhaler prior to or following the actuation and not holding the breath after inhalation.1 Face-to-face instructing of a patient on the correct use of an inhaler is required at diagnosis and at regular intervals, as well as when switching inhalers. Such regular coaching may also support active involvement of the patient and good HCP-patient partnership.1,2 Current guidelines for asthma and COPD emphasise involving the patient in their inhaler selection as patient satisfaction is one of the key aspects in ensuring treatment adherence. In addition to effectiveness, patients appreciate usability features, such as size, portability and ease of use.2 Real world studies on Easyhaler suggest that a majority of patients are satisfied with Easyhaler and consider the inhaler as easily portable as well as easy to learn and to use in everyday life.1 Several recent studies addressing a switch from another inhaler to Easyhaler have demonstrated positive results in both asthma and COPD control as well as high patient satisfaction.1
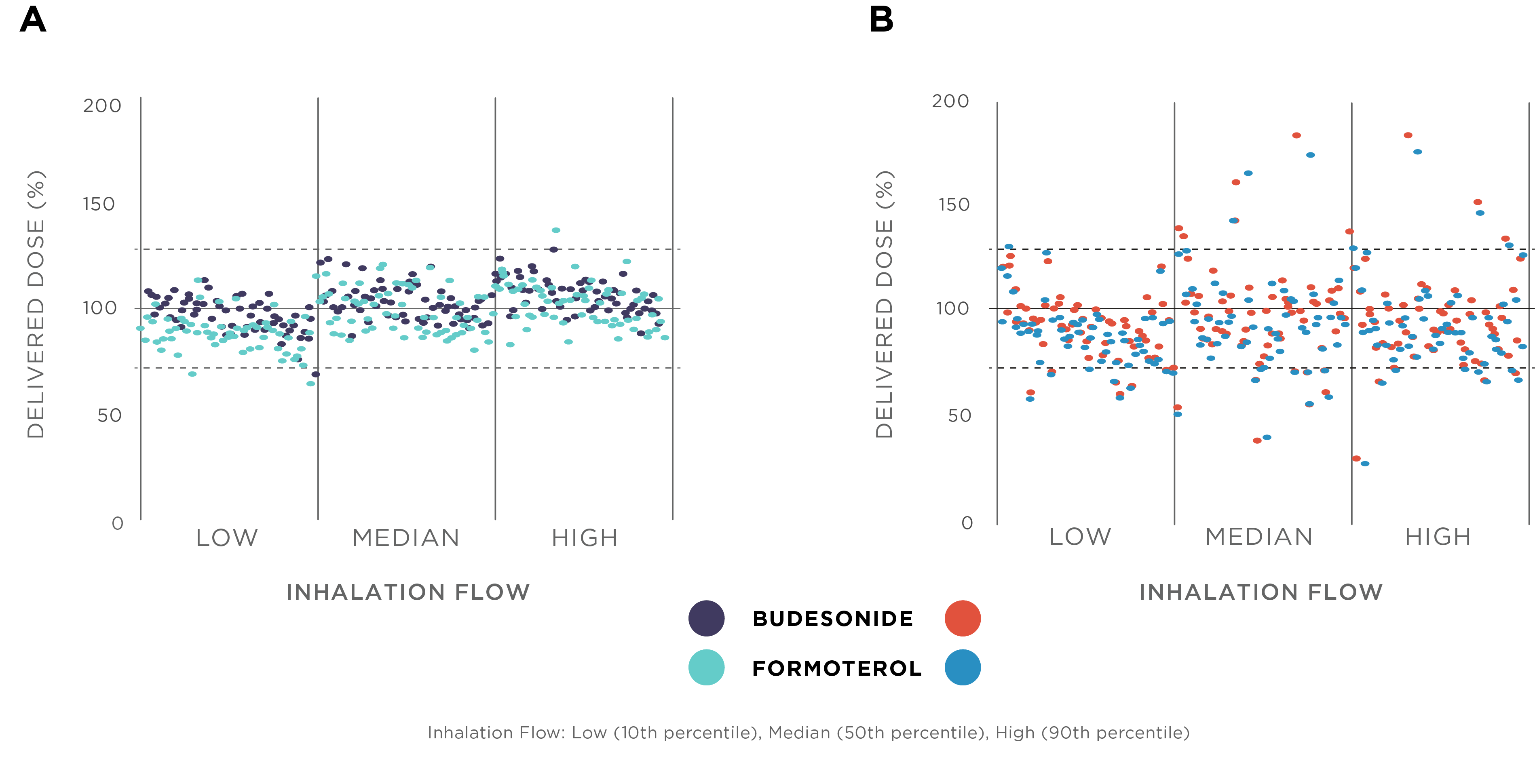
Figure 1. Comparison of Bufomix (budesonide-formoterol) Easyhaler (A) and budesonide-formoterol originator product (B) regarding the consistency of the delivered dose at different inhalation flow rates.1,2
The wide variety of inhalers in the market provides plenty of opportunities to find the optimal inhaler for each patient. The technical features of Easyhaler make it suitable for different patient groups including patients with low inspiratory effort. Patient satisfaction with Easyhaler is high and the inhaler is considered convenient and easy to use and to learn.
The availability of Easyhaler products varies between countries. Bufomix (budesonide-formoterol) Easyhaler is the only preparation available in the Republic of Ireland.
Bufomix (Budesonide/Formoterol) Easyhaler Contraindications and Undesirable Effects
Contraindications:
Hypersensitivity to the active substances or lactose monohydrate (which contains small amounts of milk protein).
Undesirable effects:
Common (≥1/100 to < 1/10): Candida infections in the oropharynx, pneumonia (in COPD patients) headache, tremor, palpitations, mild irritation in the throat, coughing, dysphonia including hoarseness.
See SmPC for full list of adverse reactions
References:
- Chrystyn H and Lavorini F. The dry powder inhaler features of the Easyhaler that benefit the management of patients. Expert Rev Respir Med. 2020, 4:1-7
- Levy M, Carroll W, Izquierdo Alonzo J, Keller C, Lavorini F, Lehtimäki L. Understanding dry powder inhalers: key technical and patient preference attributes. Adv Ther 2019, 36(10):2547-2557.
Date of preparation: June 2024 / EASYH-885(2)
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |

