Switching to the Easyhaler Device to control Asthma and COPD: A literature review
Insights | 03/12/2021Prescribing information available here and Adverse Event Reporting information can be found at the bottom of the page.
- Issues in inhaler selection and device switching, with focus on the Easyhaler® device, were summarised in a review article.1
- The evidence showed that compared to other inhalers, Easyhaler provides several benefits in the treatment of asthma and COPD:
- Easyhaler provided consistent drug delivery even at low inspiratory flow rates.
- Easyhaler was found to be easy to teach, learn, and use.
- Patients reported high satisfaction with Easyhaler and preferred it over other inhalers.
- Authors concluded that switching to an Easyhaler device to improve asthma and COPD does not incur any additional risk.
Success in treating asthma and chronic obstructive pulmonary disease (COPD) may depend on inhaler choice, as poor inhaler practice contributes to suboptimal clinical control in these diseases. In a review article published in 2021, asthma and COPD experts from various European countries addressed issues in inhaler selection and device switching, with a focus on the Easyhaler® device.1 This review concluded that Easyhaler provides several benefits for treatment of asthma and COPD.
The evidence showed that Easyhaler provides consistent drug delivery at low inhalation flow rates (30 l/min) and inhalation volumes (down to 0.5 l) which makes Easyhaler suitable for the majority of patients with asthma or COPD regardless of age or disease severity. In addition, the drug delivery performance of Easyhaler was robust throughout the inhaler’s lifespan and was unaffected by environmental stress such as dropping, vibration, moisture, and freeze-thawing.
Easyhaler therapy was also associated with improved treatment outcomes in real-life clinical practice. Easyhaler therapy resulted in improvement in lung function, disease control, and health-related quality of life in patients who switched from another inhaler to Easyhaler (Fig 1).
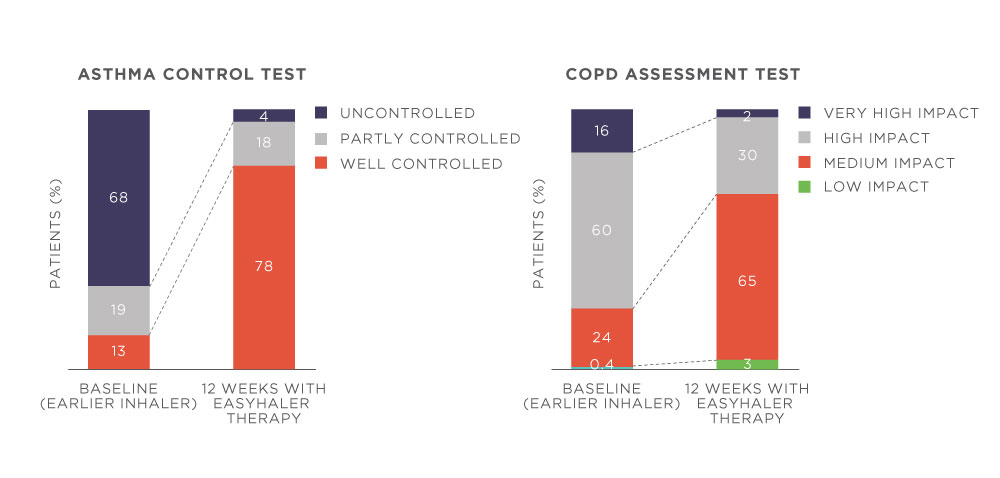
Figure 1. Switching to Easyhaler improved disease control in patients with asthma (n=398) or COPD (n=563). Patients received 12 weeks of Bufomix (budesonide/formoterol) Easyhaler Easyhaler therapy. P<0.0001 (baseline visit versus 12 weeks visit) for both asthma and COPD comparisons. Adapted from Gálffy G, et al. 2019.2
In addition, patients were more satisfied with Easyhaler and preferred it over other DPIs or other types of inhalers. Almost all patients (97.2%) reported high satisfaction with Easyhaler (Fig. 2).
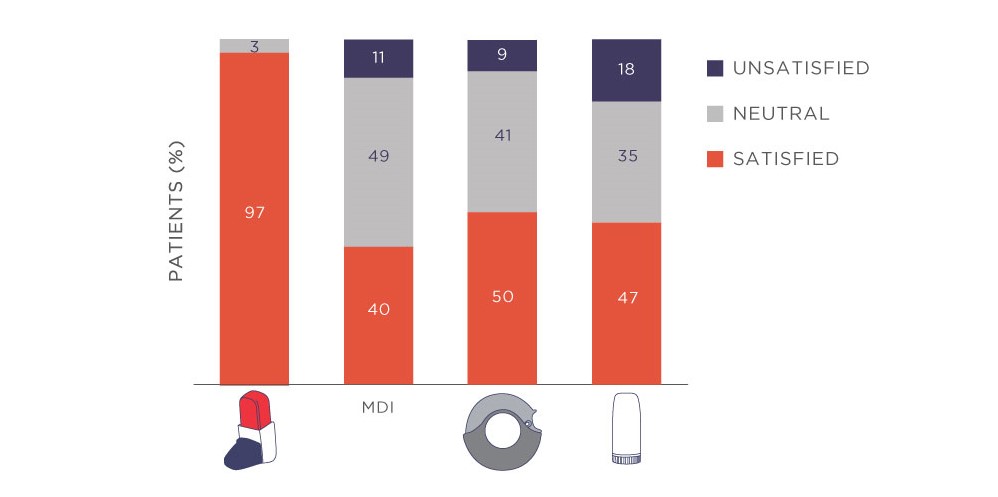
Figure 2. Almost all patients were satisfied with Easyhaler (n=621 patients with asthma, n=778 patients with COPD, n=99 ACO). Comparisons with other inhalers are shown. Adapted from Tamási L, et al. 2018.3
Furthermore, a majority of physicians (>90%) reported Easyhaler as quick and easy to teach, and patients reported Easyhaler to be easy to learn and use. Patients also made fewer critical errors with Easyhaler than with other inhalers (Fig. 3). Critical errors in inhaler use were strongly related to poor asthma control.
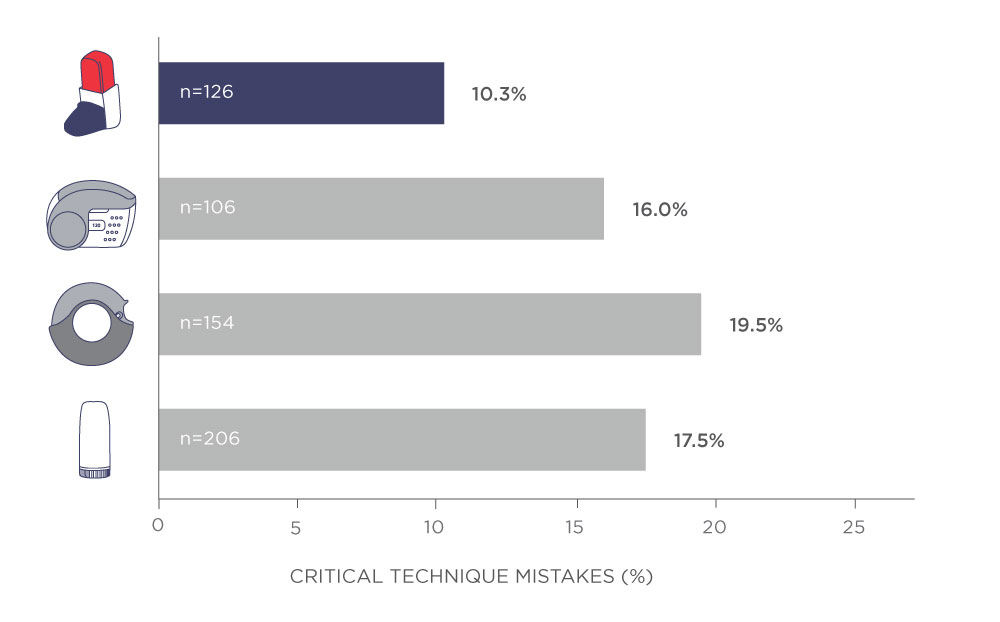
Figure 3. Significantly fewer critical mistakes were recorded among Easyhaler users versus other dry-powder inhaler users (10.3% vs 18.4%; P<0.05). Patients with asthma were referred from primary to specialist care for the first time. Adapted from Ribó P, et al. 2019.4
In addition, the review reported that the carbon footprint of DPIs such as Easyhaler is lower than the carbon footprint of MDIs which contain propellants that are powerful greenhouse gases. Switching to DPIs such as Easyhaler has been proposed as a cost-effective way to reduce emissions.
In conclusion, Easyhaler presented several benefits exemplified by consistent drug delivery across a wide range of peak inspiratory flow rates, few critical errors in use, high patient satisfaction, and a reduction in the emission of greenhouse gases. Finally, the experts concluded that switching to an Easyhaler device to control asthma and COPD does not incur any additional risk.
You can watch an animation of the results here.
References:
- Lavorini F, Chudek J, Gálffy G, Pallarés-Sanmartin A, Pelkonen AS, Rytilä P, Syk J, Szilasi M, Tamási L, Xanthopoulos A, Haahtela T. Switching to the Dry-Powder Inhaler Easyhaler®: A Narrative Review of the Evidence [published online ahead of print, 2021 Sep 27]. Pulm Ther. 2021;1-19.
- Gálffy G, Szilasi M, Tamási L. Effectiveness and patient satisfaction with budesonide/formoterol Easyhaler® among patients with asthma or COPD switching from previous treatment: A real-world study of patient-reported outcomes. Pulm Ther. 2019;5:165–77.
- Tamási L, Szilasi M, Gálffy G. Clinical effectiveness of budesonide/formoterol fumarate Easyhaler® for patients with poorly controlled obstructive airway disease: A real-world study of patient-reported outcomes. Adv Ther. 2018;35:1140–5
- Ribó P, Molina J, Calle M, et al. Prevalence of modifiable factors limiting treatment efficacy of poorly controlled asthma patients: EFIMERA observational study. NPJ Prim Care Respir Med. 2020;30:33.
Date of preparation: June 2024 / EASYH-1919(1)
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |




