Bufomix (budesonide/formoterol) Easyhaler® Maintenance and Reliever Therapy: MART
Insights | 14/03/2021Prescribing information available here and Adverse Event Reporting information can be found at the bottom of the page. Maintenance and reliever therapy is licensed for Bufomix (budesonide-formoterol) Easyhaler 80/4.5mcg and 160/4.5mcg strengths in adults and adolescents ≥12 years.1
- Poor adherence to treatment is one of the main causes of sub-optimal clinical results in the treatment of asthma.
- Maintenance and Reliever Therapy (MART) and the characteristics of an ‘ideal inhaler’ can help to simplify asthma management and improve adherence to treatment.
- MART has been demonstrated to reduce asthma exacerbations and can reach its maximum therapeutic potential when administered through an ‘ideal’ single combination inhaler.2
- Bufomix (budesonide-formoterol) Easyhaler® combines both maintenance and reliever therapy into one device, and demonstrates several characteristics of an ‘ideal inhaler’, such as reproducibility and precision.2
Poor adherence to maintenance medication, both in adults and children, is a major issue in the treatment of asthma. Patients often use maintenance medication only when symptoms occur, and suspend the medication when they perceive it to be unnecessary. At the same time, asthma guidelines and physicians usually recommend a short-acting beta-agonist (SABA) as a quick relief of asthma symptoms. However, introduction of anti-inflammatory treatment, principally inhaled corticosteroids (ICS), has also been proven to reduce the symptoms and has been associated with improved asthma control and reduction of the cost and burden of asthma. Adding ICS as needed for all patients with asthma, even for those having symptoms less than twice a month and no exacerbation risk factors, helps to reduce the risk of serious exacerbations and subsequent decline in lung function.2
The approach to combine ICS and long-acting beta-agonist (LABA) is called MART. MART is a combination of, e.g., budesonide and formoterol in a single inhaler to be used as both prevention in daily maintenance therapy and a reliever of symptoms when required. A review has confirmed that such a combination to be at least as effective in achieving asthma control as ICS/LABA plus as needed SABA, this review also mentions that As-needed budesonide/formoterol has been shown to be a more effective reliever in mild asthma than SABA alone2
Having a single inhaler that combines both maintenance and reliever therapy, instead of having multiple devices, is an advantage for asthma patients. MART in a single inhaler together with characteristics of an ‘ideal inhaler’ could address the current unmet needs in patient inhalation technique and treatment adherence, and therefore have the potential to improve patient outcomes. Bufomix Easyhaler® is a MART inhaler that possesses several characteristics of an ‘ideal inhaler’ in real-life conditions, such as effectiveness, reproducibility, precision (Figure 1), stability, and versatility. In several studies, Bufomix Easyhaler® has been referred to as easy to learn, to use and to keep clean, and also comfortable, small, portable and discreet, making it an inhaler device close to being an ‘Ideal Inhaler’.2
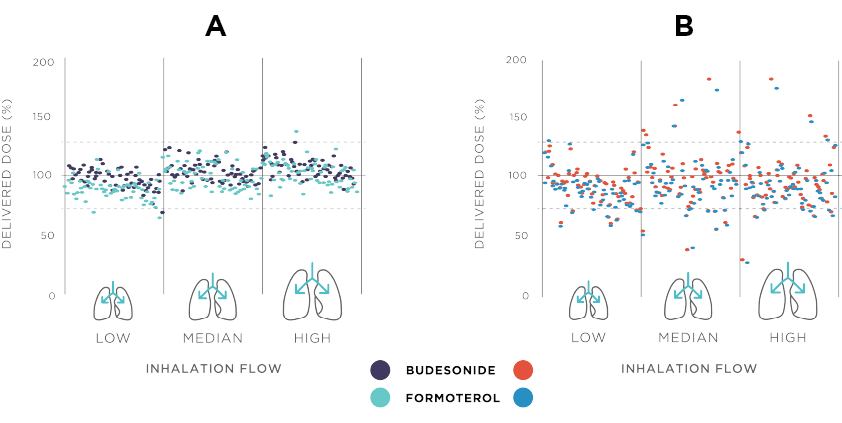
Figure 1. Comparison of Bufomix (budesonide-formoterol) Easyhaler (A) and budesonide-formoterol originator product (B) regarding the consistency of the delivered dose, at three different inhalation flow rates. Results originally published in Haikarainen et al. 2017.3 The delivered dose is expressed as a percent of the nominal labelled dose. Each data print represents a single dose actuation.
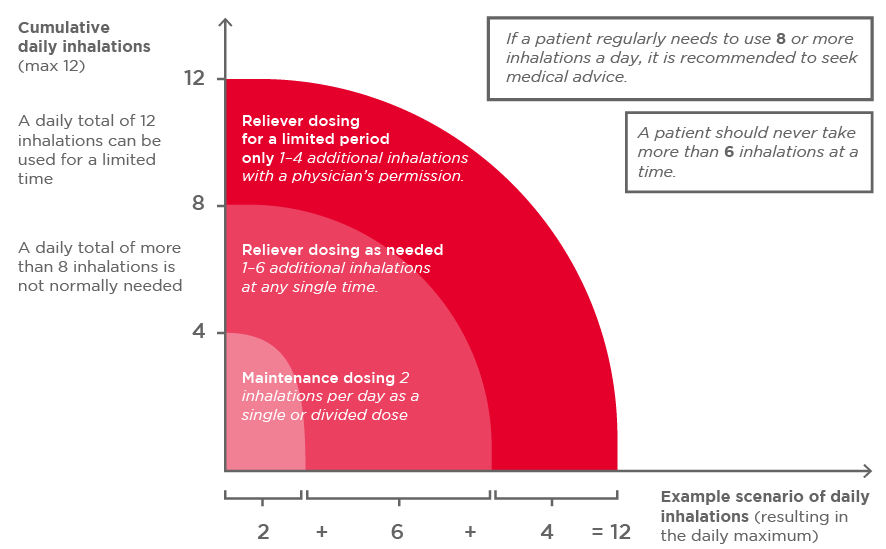
Bufomix Easyhaler is available in two different strengths for MART: 80/4.5 micrograms and 160/4.5 micrograms. MART is indicated for adults and adolescents (≥12 years) with inadequate asthma control, and in frequent need of reliever medication. The recommended maintenance dose is 2 inhalations per day, given as a single or divided dose, with additional doses of reliever medication if needed (Figure 2). The 160/4.5 micrograms strength can also be used preventatively for allergen- or exercise-induced bronchoconstriction.1
Contraindications:
Hypersensitivity to the active substances or lactose monohydrate (which contains small amounts of milk protein).
Undesirable Effects:
Common (≥1/100 to < 1/10): Candida infections in the oropharynx, pneumonia (in COPD patients) headache, tremor, palpitations, mild irritation in the throat, coughing, dysphonia including hoarseness.
See SmPC for full list of adverse reactions
References:
- Budesonide-formoterol Easyhaler 80/4.5, 160/4.5 and 320/9 mcg. SmPC. Orion Pharma.
- Di Marco, F. Today’s improvement in asthma treatment: role of MART and Easyhaler. Multidisciplinary Respiratory Medicine 2020. 15.
- Haikarainen J, Selroos O, Löytänä T, Metsärinne S, Happonen A, Rytilä P. Budesonide/Formoterol Easyhaler®: Performance Under Simulated Real-Life Conditions. Pulmonary Therapy 2017. 3, 125–138.
Date of preparation: January 2024 / EASYH-1253(2)
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |




