Patients with asthma or COPD are able to efficiently use Easyhaler® regardless of age or disease severity
Insights | 13/09/2022
Prescribing information available here and Adverse event reporting information can be found at the bottom of this page
Patients with asthma or COPD may suffer from severe airflow limitation and thus have difficulties receiving sufficient drug dosage via dry powder inhaler (DPI)
- Patients with asthma or COPD may suffer from severe airflow limitation and thus have difficulties receiving sufficient drug dosage via dry powder inhaler (DPI).
- In a recent study, almost all (>99%) studied patients with asthma or COPD, regardless of age or disease severity, were able to achieve the peak inspiratory flow (PIF), ≥30 L/min, required for efficient dose delivery through the Easyhaler®DPI.1
Inhalation via dry powder inhaler (DPI) is a common route of drug administration in the treatment of asthma and chronic obstructive pulmonary disease (COPD). However, the ability of patients to achieve the correct drug dose through their inhaler may be compromised, as some patients, especially young children and those with severely lowered lung function, may encounter difficulties in generating a forceful inhalation. Previous studies have shown that many patients are able to achieve sufficient inspiratory flow for drug delivery 2,3, but the effect of age, lung function and other patient characteristics on inspiratory flow rates have not been thoroughly investigated.
This study is a post hoc analysis1 from two pre-registration multicentre clinical studies4,5 on salmeterol-fluticasone propionate Easyhaler® and budesonide-formoterol Easyhaler®. Those studies aimed to determine the inspiratory flow capacity of asthma and COPD patients across various disease severities and age groups. The primary endpoint was peak inspiratory flow (PIF) rate through inhalation via Easyhaler®, after standard training.
Almost all patients (n=380/383) were able to generate a PIF rate ≥30 L/min through the Easyhaler®, which is considered sufficient for successful dose delivery. The mean PIF values were similar across the following groups: children (6–17 years) with asthma, adults with asthma, and patients with COPD (Figure 1).1
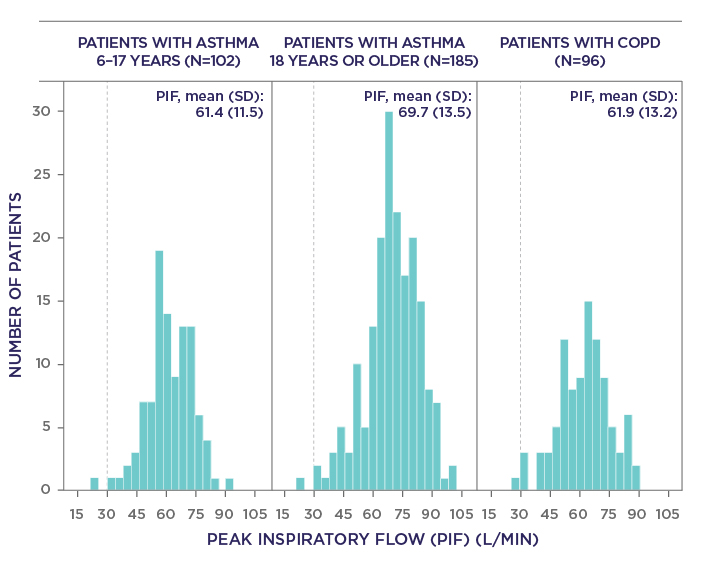
Figure 1. Distribution of peak inspiratory flow through an Easyhaler® inhaler in the per-protocol population (N=383). The dotted line indicates the PIF threshold sufficient for dose delivery. COPD, chronic obstructive pulmonary disease; PIF, peak inspiratory flow. Adapted from Malmberg et al. 20211
Additionally, correlation of PIF rate through the Easyhaler® with age, weight, height, and various lung function parameters were analysed. In children with asthma, PIF values moderately increased with age (r=0.301; p=0.0021). In adults with asthma and all patients with COPD, the association was inverse and less apparent (r=–0.212; p=0.0037 and r=–0.246; p=0.0156, respectively). PIF rate also tended to increase in correlation with greater patient height and weight.1
There was no strong correlation (defined as r≥0.50) between PIF rate through the Easyhaler® and most of the measured lung function parameters. However, a stronger association was observed between PIF rate through the Easyhaler® and native PIF in adults with asthma and patients with COPD (r=0.440 and r=0.662, respectively; both p=<0.0001). Additionally, forced inspiratory vital capacity (FIVC) was strongly associated with PIF rate through the Easyhaler® in all subgroups. This association increased with age in patients with asthma, and was highest in patients with COPD (r=0.499–0.632; p<0.001 in all subgroups).1
In conclusion, PIF rate through the Easyhaler® was found to correlate with age, height, and weight in younger patients with asthma. The observed correlations between PIF rate through the Easyhaler® and most lung functions were low. Overall, this analysis demonstrated that over 99% of all patients with asthma or COPD can achieve sufficient PIF rates (≥30 L/min) needed for the effective use of Easyhaler® for drug delivery.
Bufomix (budesonide/formoterol) Easyhaler® is the only Easyhaler range available in Ireland
Bufomix (Budesonide/Formoterol) Easyhaler Contraindications and Undesirable Effects
Contraindications:
Hypersensitivity to the active substances or lactose monohydrate (which contains small amounts of milk protein).
Undesirable effects:
Common (≥1/100 to < 1/10): Candida infections in the oropharynx, pneumonia (in COPD patients) headache, tremor, palpitations, mild irritation in the throat, coughing, dysphonia including hoarseness.
See SmPC for full list of adverse reactions
Reference:
- Malmberg, L., Pelkonen, A., Vartiainen, V., Vahteristo, M., Lähelmä, S., & Jõgi, R. (2021). Patients with asthma or chronic obstructive pulmonary disease (COPD) can generate sufficient inspiratory flows via Easyhaler® dry powder inhaler: a pooled analysis of two randomized controlled trials. J Thorac Dis, Epub ahead of print.
- Borgström L, Asking L, Thorsson L. Idealhalers or realhalers? A comparison of Diskus and Turbuhaler. Int J Clin Pract. 2005;59(12):1488–95.
- Selroos O, Borgström L, Ingelf J. Performance of Turbuhaler® in patients with acute airway obstruction and COPD, and in children with asthma: understanding the clinical importance of adequate peak inspiratory flow, high lung deposition, and low in vivo dose variability. Treat Respir Med. 2006;5(5):305–15.
- Jõgi R, Lähelmä S, Vahteristo M, et al. In vitro flow rate dependency of delivered dose and fine particle dose of salmeterol/fluticasone propionate Easyhaler and Seretide Diskus with patient flow rates collected in a randomized controlled trial. J Aerosol Med Pulm Drug Deliv 2019;32:88-98.
- Malmberg LP, Everard ML, Haikarainen J, Lähelmä S. Evaluation of in vitro and in vivo flow rate dependency of budesonide/formoterol Easyhaler®. J Aerosol Med Pulm Drug Deliv. 2014;27(5):329–40.
Date of preparation: June 2024 / EASYH-2659(1)
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |



