Switching to
Bufomix (budesonide/formoterol) Easyhaler® may improve disease control and health related quality of life
Insights |
19/09/2022
Prescribing information available here and Adverse Event Reporting information can be found at the bottom of the page
Non-inferiority of asthma control was assessed among 117 adult patients switching to Bufomix (budesonide/formoterol)Easyhaler® from the budesonide/formoterol originator product in a real-life prospective, observational study.
Syk et al. 2019. Adv Ther
- Non-inferiority of asthma control was assessed among 117 adult patients switching to Bufomix Easyhaler® from the Budesonide/Formoterol originator product in a real-life prospective, observational study.
- Switching to Bufomix Easyhaler® resulted in an improvement in asthma control and health-related quality of life.
- Physicians and patients regarded Bufomix Easyhaler® as easy to teach, learn and use, and the majority of patients mastered the inhaler technique in less than 5 minutes.
In the current practice of asthma treatment, physicians are often cautious with switching between inhaler devices due to the potential risk of impaired asthma control or an increase in exacerbations. In order to overcome such limitations in treatment optimisation, real-life studies are required to compare the effectiveness, ease of use, and patient’s or physician’s preferences between different inhalers. In the present real-life study, the maintenance of asthma control (i.e. non-inferiority) was evaluated in asthmatic patients switching to Bufomix Easyhaler® from the Budesonide/Formoterol originator product in Swedish primary care.
A total of 117 adult patients with persistent asthma were included in this non-interventional, single arm multicentre study. The primary endpoint was to evaluate the non-inferiority of asthma control when switching from budesonide/formoterol originator (160/4.5 mcg or 320/9.0 mcg, use ≥ 6 months) to an equivalent dose Bufomix Easyhaler®, using the Asthma Control Test (ACT). In addition, a mini-asthma quality of life questionnaire (mini-AQLQ), spirometry (FEV1, FVC), as well as learning and usage questionnaires were utilized to assess the asthma-related quality of life, lung function and patient/physician perspectives, respectively. The baseline assessments and the guided switch to Bufomix Easyhaler® were performed at the first visit to the primary care (baseline) and the outcomes were evaluated after 12 weeks (±2 weeks) of Bufomix Easyhaler® treatment.
Switching to Bufomix Easyhaler® therapy improved disease control, as demonstrated by an increase in ACT score (from 18.9 to 20.7; P<0.0001). Importantly, such a change in the ACT score clearly fulfils the non-inferiority criteria. Furthermore, the proportion of patients with well-controlled asthma increased from 53% to 70.9% during the 12 weeks of Easyhaler therapy (Figure 1). Switching to Bufomix Easyhaler® also improved patients’ asthma-related quality of life (mini-AQLQ score increase from 5.4 to 5.8; P<0.0001). Notably, the change in inhaler device did not impair the lung function, which remained stable or even improved slightly throughout the treatment period. Physicians and patients regarded Bufomix Easyhaler® as easy to teach, learn and use, and the majority of patients (82%) mastered the correct use of the inhaler in less than 5 minutes.
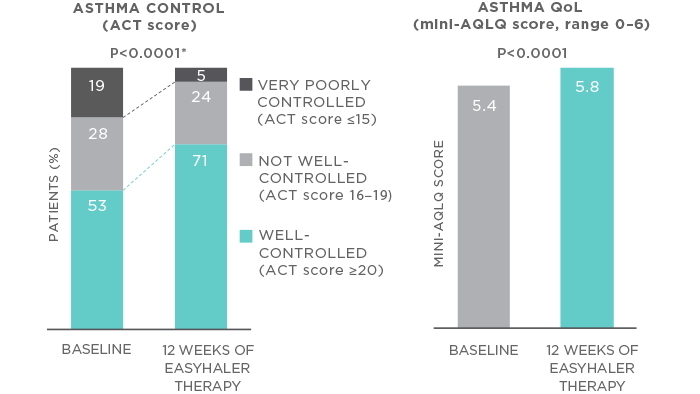
Figure 1. Switching to Bufomix (budesonide/formoterol) Easyhaler® improves asthma control and health-related quality of life among adult patients with persistent asthma. N=117 patients switching to Bufomix Easyhaler® from the equivalent dose Budesonide/Formoterol originator. *P<0.0001 for change in average ACT score. Patients received 12 weeks of Bufomix Easyhaler® therapy.
In conclusion, the results indicate that switching to Bufomix Easyhaler from the equivalent dose budesonide/formoterol originator product is clinically effective and may even lead to improved asthma control and health-related quality of life.
Reference:
Syk J, Vinge I, Sörberg M, Vahteristo M, Rytilä P. A Multicenter, Observational, Prospective Study of the Effectiveness of Switching from Budesonide/Formoterol Turbuhaler® to Budesonide/Formoterol Easyhaler®. Adv Ther 2019. [Epub ahead of print]
Date of preparation: March 2024 / EASYH-2652(1)
Bufomix Easyhaler Contraindications and Undesirable effects
Contraindications: Hypersensitivity to the active substances or lactose monohydrate (which contains small amounts of milk protein).
Undesirable effects:
Common (≥1/100 to < 1/10): Candida infections in the oropharynx, pneumonia (in COPD patients) headache, tremor, palpitations, mild irritation in the throat, coughing, dysphonia including hoarseness.
See SmPC for full list of adverse reactions
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |

