The importance of patient satisfaction, preference, and adherence to inhaler for long-term asthma control
Insights | 05/06/2021Prescribing information available here
- Patient satisfaction with an inhaler is associated with higher adherence and improved asthma control.
- Easyhaler® achieved the highest scores for patient satisfaction and preference, compared to other DPIs in two recent observational real-world studies in adult patients with persistent bronchial asthma.2,3
Lack of adherence to treatment has adverse consequences on asthma control. The correct choice of inhaler device can improve patient satisfaction with treatment and increase adherence, leading to more favourable clinical outcomes. Recent studies have explored this topic and offer insights on patient satisfaction with inhalers, and how it affects adherence to treatment and asthma control.
The Asthma Satisfaction, Control and Adherence (ASCONA) study was a cross-sectional, observational real-world study conducted in 59 hospitals across Spain.1 The aim of this study was to evaluate the effect of patient satisfaction with their inhaler on adherence and asthma control. Adult patients with moderate to severe asthma who had been treated with the same inhaler for at least 3 months prior to inclusion were recruited (n=778). The study revealed that patient satisfaction with an inhaler is associated with higher adherence and improved asthma control.1
In a sub-analysis of the ASCONA study (n=328), patient satisfaction with their current inhaler was evaluated for each device separately. Easyhaler® achieved the highest score for satisfaction compared with other dry powder inhaler devices.2
In the DPI PREFER study, inhaler preference and satisfaction were explored after patient switching to Easyhaler® from another dry powder inhaler (DPI) device.3 Adult patients with persistent bronchial asthma (n=502) were recruited to the multicentre, non-interventional, single-visit, observational study. The patients had been receiving inhaled corticosteroids and long-acting beta-2 agonists through a DPI device other than Easyhaler® for at least 3 months and were referred for the first time to an allergist or pulmonologist. Adherence to current treatment, as measured by the Test of Adherence to Inhalers questionnaire (TAI), was poor in 43.5% of patients who completed the test (n=485). After filling the questionnaires regarding their current inhaler, the patients were trained to use the Easyhaler® device.
Patient satisfaction was assessed with an adapted Feeling of Satisfaction with Inhaler questionnaire (FSI-10). The adapted version excluded questions regarding long-term use of an inhaler. Satisfaction with Easyhaler® was significantly higher than with the previous inhaler, 31.8±3.24 points vs. 29.12±5.15 points, p<0.001 (n=485). Statistical analysis using Pearson’s chi-square tests showed that satisfaction with the previous inhaler was significantly associated with adherence level (p<0.001), asthma control (p<0.001), and even pulmonary function (p=0.006). Accordingly, low satisfaction with the previous inhaler was significantly associated with poor asthma control and poor adherence. Low satisfaction with the previous inhaler was also significantly associated with the inhaler type (p<0.001).
The patients also completed a 4-item self-perception questionnaire to evaluate their inhaler device preference. The results showed that 38.4% of the patients preferred Easyhaler® exclusively, and 45.8% of the patients reported a similar preference for both inhalers (previous and Easyhaler®) (Figure 1). Only 15.3% of patients exclusively preferred the previous DPI device. From a practical perspective, the results suggest that 84.2% of patients would accept the switch to Easyhaler®.
The factors affecting device preference were further studied by multivariate logistic regression analysis. The results showed that the FSI-score, measuring satisfaction with the inhaler, was associated with device preference: a high FSI-10 score for Easyhaler® strongly predicted preference for it, with an odds ratio of 1.79 (95% CI 1.52–2.11). On the other hand, a high FSI-10 score for the previous inhaler favoured preference for that one (odds ratio 0.71, 95% CI 0.63–0.80).
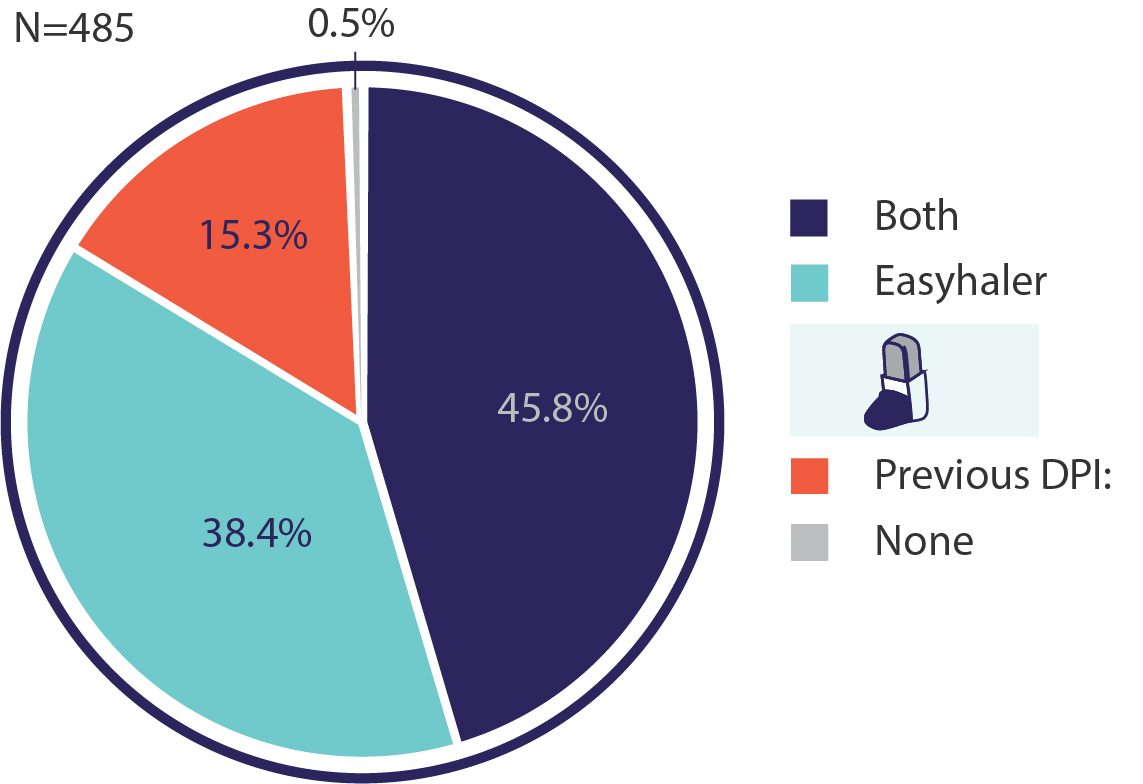
Figure 1. Device preference according to a self-perception questionnaire in DPI PREFER study (n=485).3
Together these three publications highlight the importance of patient opinion in the clinical care of asthma: patients who are satisfied with their inhaler tend to prefer using it, adhere to the treatment, and achieve better asthma control (Figure 2). Conversely, patients who are not satisfied with their inhaler tend to have worse treatment adherence and thus worse asthma control.1 Therefore, it is important to regularly follow up on patient satisfaction with the current inhaler. This will allow identification of the patients that are not satisfied, who could then be offered other options to improve adherence. According to the GINA guidelines, patient preference should be regularly assessed in control-based asthma management.4
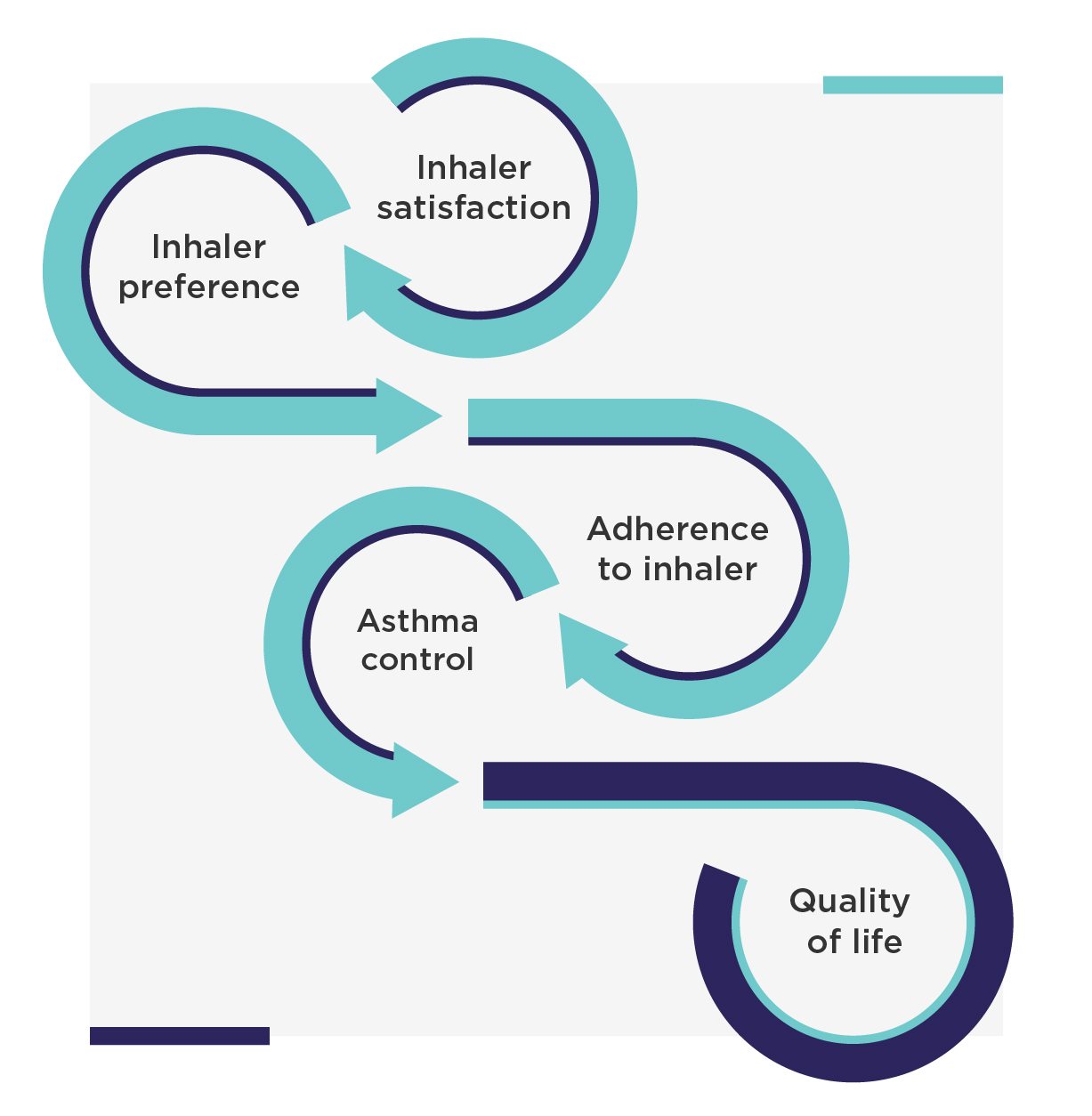
Figure 2. Pathway from patient inhaler satisfaction to good asthma control and quality of life
The studies showed that patients are more satisfied with Easyhaler® over various other DPI devices, both in the setting of Easyhaler® being their current inhaler or when evaluating the opinion of the patients currently using another device. Patients also preferred Easyhaler® more often than their current inhaler. Easyhaler® is therefore a prominent inhaler option.
For further insights on patient preference and satisfaction, see:
References:
- Plaza V, Giner J, Calle M, Rytilä P, Campo C, Ribó P, Valero A. Impact of patient satisfaction with his or her inhaler on adherence and asthma control. Allergy Asthma Proc. 2018 Nov 18;39(6):437-444.
- Valero A, Ribó P, Maíz L, Barbero E, Calle M, Campo C, Rytilä P, Giner J, Plaza V. Asthma patient satisfaction with different dry powder inhalers. Expert Rev Respir Med. 2019 Feb;13(2):133-138.
- Alvarez-Gutiérrez FJ, Gómez-Bastero Fernández A, Medina Gallardo JF, Campo Sien C, Rytilä P, Delgado Romero J. Preference for Easyhaler® Over Previous Dry Powder Inhalers in Asthma Patients: Results of the DPI PREFER Observational Study. Patient Prefer Adherence. 2021;15:349-358.
- 2023 GINA Main Report - Global Initiative for Asthma - GINA (ginasthma.org)
Date of preparation: December 2023 / EASYH-1556(2)
| Adverse effects should be reported. You can report side effects directly via the Health Products Regulatory Authority (HPRA) website: www.hpra.ie or by email on medsafety@hpra.ie. Adverse effects should also be reported to Orion Pharma via ie.medicalinformation@orionpharma.com |

