Pharmacokinetics of salmeterol and fluticasone propionate delivered in combination via Easyhaler and Diskus dry powder inhalers in healthy subjects
Insights | 26 November 2018Study summary:
Pharmacokinetics of salmeterol and fluticasone propionate delivered in combination via Easyhaler® and Diskus® dry powder inhalers in healthy subjects.
Kirjavainen et al. 2018. J Aerosol Med Pulm Drug Deliv
Salmeterol/fluticasone Easyhaler combination therapy was developed to further complete the Easyhaler product family offering and to support the treatment of asthma and COPD patients.
In the study by Kirjavainen et al., the therapeutic equivalence between Salmeterol/fluticasone Easyhaler, and an originator product, Seretide® Diskus® was demonstrated. The primary pharmacokinetic objectives were to indicate noninferiority in total systemic exposure (safety) and bioequivalence in lung deposition (efficacy) between Easyhaler and the originator product. Additionally, the clinical safety of both Salmeterol/fluticasone Easyhaler and Seretide Diskus was assessed (Figure 1).
Figure 1. Determination of therapeutic equivalence between Salmeterol/fluticasone Easyhaler® and the originator product Seretide® Diskus®. Study design and primary variables.
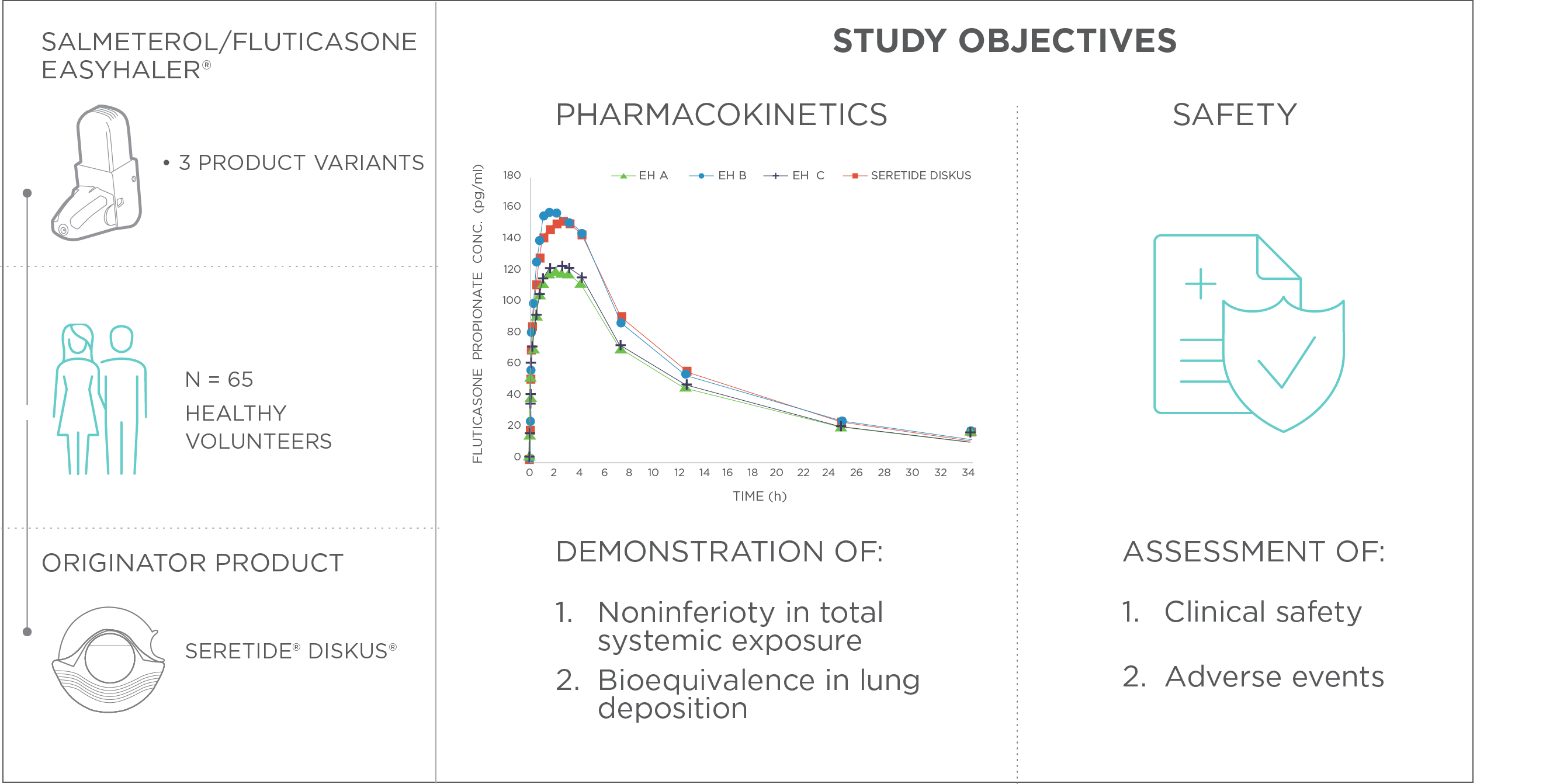
The study was conducted as an open and randomized, single-dose pharmacokinetic study in a single center. A total of 65 healthy volunteers participated in the study. Seretide Diskus (50/500 µg per inhalation) was used as the reference product, whereas three different product variants were tested for Salmeterol/fluticasone Easyhaler (50/500 µg per inhalation).
The primary variables for the assessment of total systemic exposure and lung deposition of fluticasone propionate were the maximum concentration of the concentration-time curve (Cmax) and the area under the concentration-time curve until the last sample with quantifiable concentration (AUCt). For salmeterol, Cmax and AUCt were the primary variables for total systemic exposure, and Cmax and AUC30min for lung deposition. Supine heart rate, blood pressure, and electrocardiogram measurements, as well as physical examination and laboratory safety assessments, were performed to determine clinical safety. Adverse events were followed throughout the whole study.
The results showed that one of the Easyhaler products was both bioequivalent in lung deposition and noninferior in total systemic exposure with the originator product. Thus, Easyhaler (Salmeterol/fluticasone Easyhaler) fulfilled the criteria for bioequivalence with the originator product. Moreover, the safety profiles of Salmeterol/fluticasone Easyhaler and the originator product were similar.
Kirjavainen M, Mattila L, Vahteristo M, Korhonen J, Lähelmä S. Pharmacokinetics of salmeterol and fluticasone propionate delivered in combination via Easyhaler and Diskus dry powder inhalers in healthy subjects. J Aerosol Med Pulm Drug Deliv 2018. doi: 10.1089/jamp.2017.1437. [Epub ahead of print]

