Patients with chronic obstructive pulmonary disease are able to reach sufficient peak inspiratory flow rate for Easyhaler®: a combined analysis from three clinical studies
Publikationer | 2024-04-08
A pooled analysis of three clinical studies showed that the clinical characteristics of impaired expiratory lung function of patients with chronic obstructive pulmonary disease (COPD) do not predict their ability to use dry powder inhalers (DPIs). A sufficient peak inspiratory flow rate (PIF) through the Easyhaler® high resistance DPI was achieved by 99% of patients with COPD.1
- Environmental considerations support the use of DPIs instead of pressurised metered dose inhalers (pMDIs), but there are concerns regarding the ability of patients with COPD to inhale efficiently with DPIs.
- In a pooled analysis of three clinical studies involving 246 patients with COPD, a sufficient PIF (at least 30 L/min) through an Easyhaler DPI was achieved by 99% of patients.
- Clinical characteristics of patients, including lung function parameters, did not predict PIF values, indicating that they do not limit the use of Easyhaler in patients with COPD.1
In the treatment of pulmonary diseases, climate concerns have led to an increasing pressure to use DPIs instead of pMDIs for drug administration. pMDIs have a 20–40- fold higher carbon footprint than DPIs, mainly due to propellants used for aerosolizing the drug formulation — DPIs use the energy of the patient’s inspiratory flow effort for this purpose. Due to these technical differences, the requirements for correct inhalation technique between these two inhaler types differ: pMDIs require coordination between the actuation of the device and precise inhalation pattern, whereas DPIs require sufficient PIF to enable accurate and consistent drug delivery to lungs. There are persistent concerns regarding the ability of patients with COPD to achieve a sufficient PIF through DPIs.
The aims of the study were to find possible clinical predictors for PIF and to assess the ability of patients with COPD to generate sufficient PIF through a high resistance DPI, Easyhaler®.1
Data from three clinical studies were combined: two randomised, multicentre, crossover, open-label studies carried out in Estonia and Finland, as well as one investigator-initiated trial.2–4 Patients with documented COPD were included in this study (N=246).1 The patients’ demographic data, PIF through Easyhaler, and other inspiratory parameters measured by spirometry were pooled (Figure 1). The patients received training for the inhaler use during the study.
The mean age of the patients was 66.8 years, and 35% of them were female. The mean PIF through Easyhaler was 56.9 L/min, and almost all patients (99%) were able to achieve at least 30 L/min PIF through Easyhaler, which is considered the limit for successful use.
To find possible predictors of PIF, univariate models were built from age, body mass index (BMI), and lung function parameters. The best degrees of determination (R2) were with peak expiratory flow (PEF), forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC), explaining 14%, 13% and 13% of variation seen in PIF through Easyhaler, respectively. A linear regression model was built using FEV1, age, gender, and BMI. In the model, FEV1 and female gender were statistically significant in predicting low PIF, but the degree of determination was only 18%. In general, the expiratory parameters were poorly associated with the inspiratory performance.
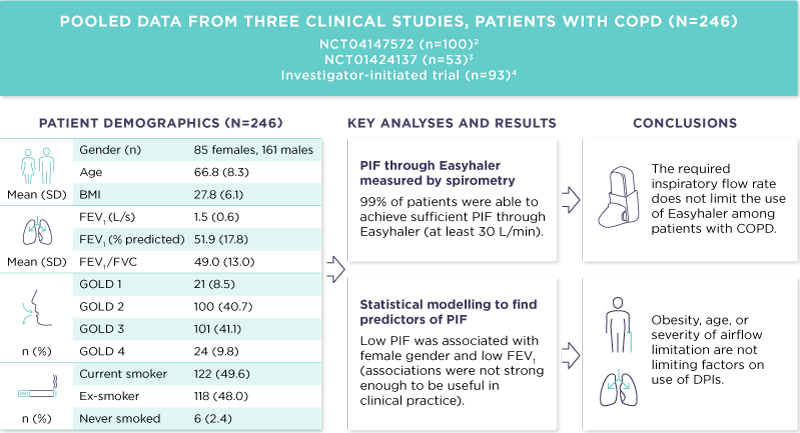
Figure 1. Summary of the study.1
BMI, body mass index; DPI, dry powder inhaler; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Pulmonary Disease; SD, standard deviation. GOLD classification of airflow obstruction: GOLD 1 mild (FEV1 ≥ 80% predicted), GOLD 2 moderate (50% ≤ FEV1 < 80% predicted), GOLD 3 severe (30% ≤ FEV1 < 50% predicted), GOLD 4 very severe (FEV1 < 30% predicted).
Based on the data, obesity, age, or severity of airflow limitation are not limiting factors on use of DPIs. Expiratory lung function parameters (e.g., FEV1) should not be used as a decisive factor when selecting an inhaler device for the patient. Failing to achieve sufficient PIF through the Easyhaler is rare, and the required PIF does not limit the use of the Easyhaler among patients with COPD. Of note, there are two variants of the Easyhaler: the standard Easyhaler is used in products with a single active ingredient, and the center-slot Easyhaler used in combination products. The standard Easyhaler was used in this study. As the center- slot Easyhaler has lower resistance, the same patient would achieve higher inspiratory flow rate through center-slot Easyhaler.1
References:
- Kainu A, Vartiainen VA, Mazur W, Hisinger-Mölkänen, H, Lavorini, F, Janson, C, Andersson, M. Successful Use of Easyhaler® Dry Powder Inhaler in Patients with Chronic Obstructive Pulmonary Disease; Analysis of Peak Inspiratory Flow from Three Clinical Trials. Pulm Ther. 2024;10(1):133-142.
- Jõgi R, Mattila L, Vahteristo M, Takala, A, Lähelmä, S, Vartiainen, VA,
Lindqvist, A. Inspiratory Flow Parameters Through Dry Powder Inhalers in Healthy Volunteers and Patients with Chronic Obstructive Pulmonary Disease (COPD): Device Resistance Does Not Limit Use in COPD. Int J Chron Obstruct Pulmon Dis. 2021;16:1193-1201. - Jõgi R, Lähelmä S, Vahteristo M, Happonen A, Haikarainen J. In Vitro Flow Rate Dependency of Delivered Dose and Fine Particle Dose of
Salmeterol/Fluticasone Propionate Easyhaler and Seretide Diskus with Patient Flow Rates Collected in a Randomized Controlled Trial. J Aerosol Med Pulm Drug Deliv. 2019;32(2):88-98. - Malmberg LP, Rytilä P, Happonen P, Haahtela T. Inspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: age and gender rather than severity matters. Int J Chron Obstruct Pulmon Dis. 2010;5:257-262.
Minimiinformation
Salflumix Easyhaler® (salmeterol/flutikasonpropionat) [Rx] F. Astma: Inhalationspulver i styrkorna 50/250 mikrogram och 50/500 mikrogram. För regelbunden behandling av bronkialastma hos vuxna och ungdomar 12 år och äldre, när kombinationsbehandling (långverkande ß2-agonist och inhalationssteroid) är lämplig för: patienter som inte uppnår adekvat symtomkontroll med inhalationssteroid och ’vid behovs’ medicinering med inhalerad kortverkande ß2-agonist eller patienter som redan har adekvat symtomkontroll med inhalationssteroid och långverkande ß2-agonist. KOL: Inhalationspulver i styrkan 50/500 mikrogram. Salflumix Easyhaler är indicerad för symtomatisk behandling av patienter från 18 år med KOL med ett FEV1 <60% av beräknat normalvärde (före bronkdilaterare) och med upprepade försämringsepisoder i sjukdomshistorien samt betydande symtom trots regelbunden behandling med bronkdilaterare. Senaste översyn av produktresuméer: 2022-12-08. Bufomix Easyhaler® (budesonid/formoterol) [Rx] F. Astma: Inhalationspulver i styrkorna 80/4,5 mikrogram, 160/4,5 mikrogram och 320/9 mikrogram. För behandling av astma när kombinationsbehandling är lämplig. Bufomix Easyhaler är inte avsett som initial astmabehandling. Bufomix Easyhaler 80/4,5 mikrogram är godkänd från 6 år vid underhållsbehandling och från 12 år för underhållsbehandling och vidbehovsbehandling. Bufomix Easyhaler 160/4,5 mikrogram är godkänd från 12 år vid underhållsbehandling och för vidbehovsbehandling. Bufomix Easyhaler 320/9 mikrogram är godkänd från 12 år vid underhållsbehandling. Vid anfallskupering ska en snabbverkande bronkdilaterare användas. KOL: Inhalationspulver i styrkorna 160/4,5 mikrogram och 320/9 mikrogram. För symtomatisk behandling av patienter med svår KOL (FEV1 <70% av förväntat värde) och tidigare upprepade exacerbationer och som har signifikanta symtom trots regelbunden behandling med bronkdilaterare. Bufomix Easyhaler 160/4,5 mikrogram och 320/9 mikrogram är godkänd från 18 år. Senaste översyn av produktresuméer: 2022-10-21. Buventol Easyhaler® (salbutamol) [Rx] F. Inhalationspulver 100 mikrogram/dos och 200 mikrogram/dos. För symtomlindring av bronkkonstriktion vid astma och kroniskt obstruktiv lungsjukdom (KOL). Patienter som ordineras regelbunden antiinflammatorisk behandling (t.ex. inhalerade kortikosteroider) ska uppmanas att fortsätta ta sin antiinflammatoriska behandling även när symtomen minskar och de inte behöver Buventol Easyhaler. Buventol Easyhaler godkänd från 6 år. Senaste översyn av produktresumé: 2024-01-25. Giona Easyhaler® (budesonid) [Rx] F. Inhalationspulver 100 mikrogram/dos, 200 mikrogram/dos och 400 mikrogram/dos. För behandling av lindrig, måttlig och svår ihållande astma. Giona Easyhaler är inte lämplig för behandling av akuta astmaanfall. Giona Easyhaler är godkänd från 6 år. Senaste översyn av produktresumé: 2022-03-17. Beclomet Easyhaler® (beklometason) [Rx] F. Inhalationspulver 200 mikrogram/dos. För behandling av bronkialastma. Beclomet Easyhaler godkänd från 5 år. Senaste översyn av produktresumé: 2021-03-04. För priser och ytterligare information se www.fass.se.

