The Internal resistance of Easyhaler®
Insights | 12/03/2020Prescribing information and Adverse event reporting can be found at the bottom of this page.
__________________________________________________________________
THE INTERNAL RESISTANCE OF AN INHALER PLAYS
A CRUCIAL ROLE IN DOSE DELIVERY
__________________________________________________________________
WATCH EASYHALER® IN ACTION
In addition to medication and dosage, the technical characteristics of a dry powder inhaler (DPI) are of importance when choosing the most suitable treatment for a patient.1,2 Importantly, the internal resistance of an inhaler plays a crucial role in dose delivery.
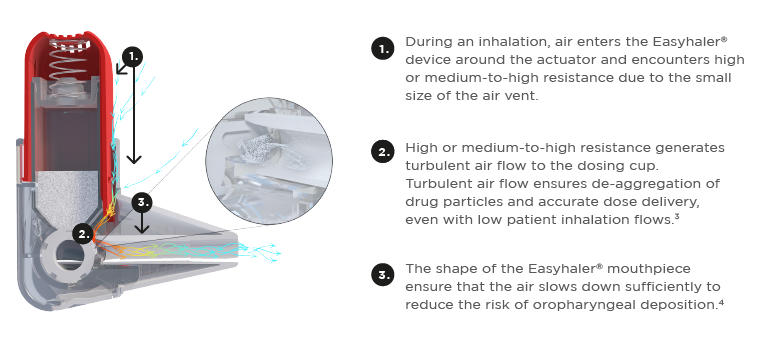
Figure 1. High-resistance DPI in action. High internal resistance and the generation of turbulent air flow inside a high-resistance dry powder inhaler (Easyhaler®). *Easyhaler® monotherapy is a high-resistance inhaler and Easyhaler® combination therapy is a medium-to-high resistance inhaler5.
__________________________________________________________________
HIGH INTERNAL RESISTANCE SUPPORTS GREATER LUNG DEPOSITION THAN LOW INTERNAL RESISTANCE1
__________________________________________________________________
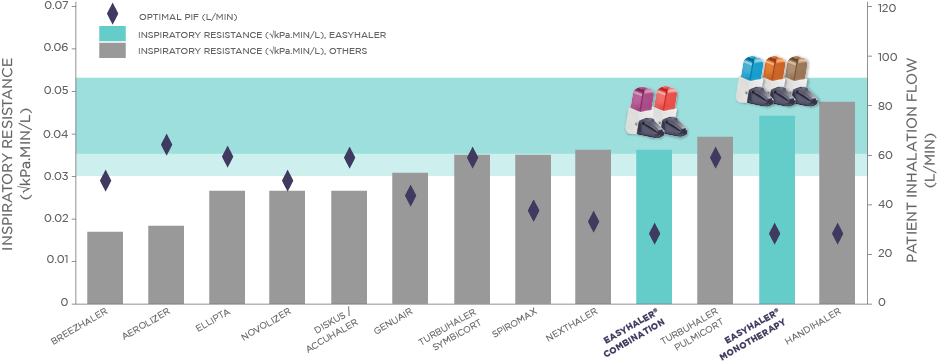
DPIs with high internal resistance generally provide greater formulation de-agglomeration and lung deposition than devices with low internal resistance.1 Inhalation against high resistance further opens the upper airways, which assists the delivery of medication into the lungs.7 Furthermore, high resistance DPI's generally require a lower inhalation flow than low resistance DPI's to provide adequate de-agglomeration and lung deposition.2 Patients with COPD, and asthmatic children with exacerbations, were able to use high resistance DPIs effectively.7 Among COPD patients, the vast majority (98%) were able to achieve a sufficient inhalation flow through the high resistance DPI (Easyhaler®) for optimal drug delivery.6 High internal resistance is therefore an important technical feature of a DPI.1
Figure 3. Adapted from Levy ML, Carroll W, Izquierdo Alonzo JL, Keller C, Lavorini F, Lehtimäki L. Understanding dry powder inhalers: key technical and patient preference attributes. Adv Ther 019;36(10):2547–57.8
DPI = Dry Powder Inhaler, pMDI = Pressurised Metered dose Inhaler
**This article refers to UK brand names. Within the clinical papers, the brand names reflect the location of where the studies took place
REFERENCES:
- Azouz W, Chetcuti P, Hosker H, Saralaya D, Stephenson J, Chrystyn H. The inhalation characteristics of patients when they use different dry powder inhalers. J Aerosol Med Pulm Drug Deliv 2015;28(1):35-42.
- Chrystyn H, Haahtela T. Real-life inhalation therapy – inhaler performance and patient education matter. Eur Respir Dis 2012;8(1):11-18.
- Capstick TGD et al Demystifying Dry Powder Inhaler Resistance with Relevance to Optimal Patient Care. Clin Drug Investig. 2024 Feb;44(2):109-114. doi: 10.1007/s40261-023-01330-2. Epub 2024 Jan 10. PMID: 38198116; PMCID: PMC10834657
- Vartiainen VA et al High inhaler resistance does not limit successful inspiratory maneuver among patients with asthma or COPD. Expert Opin Drug Deliv. 2023 Mar;20(3):385-393. doi: 10.1080/17425247.2023.2179984. Epub 2023 Feb 26. PMID: 36820500.
- Haughney et al 2021 Peak Inspiratory Flow Measured at Different Inhaler Resistances in Patients with Asthma.
- Malmberg L, Rytilä P, Happonen P, Haahtela T. Inspiratory flows through dry powder inhaler in chronic obstructive pulmonary disease: age and gender rather than severity matters. Int J of Chron Obstruct Pulmon Dis 2010;5:257–62.
- Demoly P, Hagedoorn P, de Boer A, Frijlink H. The clinical relevance of dry powder inhaler performance for drug delivery. Respiratory Medicine 2014;108(8):1195–1203.
- Levy ML, Carroll W, Izquierdo Alonzo JL, Keller C, Lavorini F, Lehtimäki L. Understanding dry powder inhalers: key technical and patient preference attributes. Adv Ther 2019;36(10):2547–57.
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Orion Pharma (UK) Ltd on
01635 520300.
August 2024 / RESP-330bdi(4)