Clinical effectiveness of Fobumix Easyhaler® (budesonide/formoterol fumarate dihydrate) for patients with poorly controlled obstructive airway disease: a real-world study of patient-reported outcomes1
Insights | 19/12/2022Prescribing Information and Adverse Event Reporting available at the bottom of the page.
- The clinical effectiveness and patient satisfaction of budesonide/formoterol dry powder inhaler Fobumix Easyhaler® was evaluated in patients with asthma, chronic obstructive pulmonary disease (COPD), and asthma, COPD overlap (ACO) in daily clinical practice.
- The use of Fobumix Easyhaler® significantly improved disease control, quality of life, and lung function in all patient groups when switched from MDI, Turbohaler® or Accuhaler® to Easyhaler®(P≤0.002).
- Patients were satisfied with the use of Fobumix Easyhaler®, and most patients learned to use it in less than 5 minutes.
Inhalation is the most recommended route for administering medication for asthma and COPD patients. Therefore, inhalation technique and inhaler device are important factors in achieving an effective clinical response. Here, the effectiveness of Fobumix Easyhaler® was assessed in a 12-week real-world, multicenter, open-label, non-randomised, non-interventional study among 1498 patients with asthma, COPD, or ACO in Hungary. The majority of patients were switched to Easyhaler® from other inhalers, most commonly from metered dose inhaler (MDI), Turbohaler®*, or Accuhaler®*.
Clinical effectiveness in patients with asthma or ACO (N=720) was evaluated based on disease control (Asthma Control Test, ACT), quality of life (mini-Asthma Quality of Life Questionnaire, mini-AQLQ), and lung function (forced expiratory volume in 1s, FEV1% predicted measurement). Similarly, in the COPD and ACO patient group (N=877), disease control was evaluated by COPD assessment test (CAT), quality of life based on the modified Medical Research Council (mMRC) dyspnea scale, and lung function by FEV1% predicted measurements. Satisfaction among patients who switched from other inhalers (N=1043) was recorded by a questionnaire in a scale from 1 (very good) to 6 (unsatisfying).
The results showed that the use of Fobumix Easyhaler® significantly improved disease control as demonstrated by the changes in the mean ACT score (from 14.2 to 21.0, P<0.001) and CAT score (from 24.2 to 18.2, P<0.001) when switched from MDI, Turbohaler® or Accuhaler® to Easyhaler® (Figure 1). Treatment with Fobumix Easyhaler® also significantly improved the quality of life in all patient groups. In addition, the use of Fobumix Easyhaler resulted in significant improvement in lung function across all patient groups (P<0.001), and importantly, also in patients who were switched from previous inhalers (P<0.001).
After 12 weeks of treatment with Fobumix Easyhaler®, the majority (87.2%) of asthma patients were able to reduce the use of a reliever inhaler. More than 90% of physicians considered Fobumix Easyhaler® as very easy or easy to teach and use, and most patients (73.8%) learned the technique in less than 5 minutes. Notably, 72.4% of the patients rated Fobumix Easyhaler® as “very good”, whereas corresponding results for MDI, Turbohaler®*, and Accuhaler®* were 12.7%, 17.3%, and 18%, respectively.
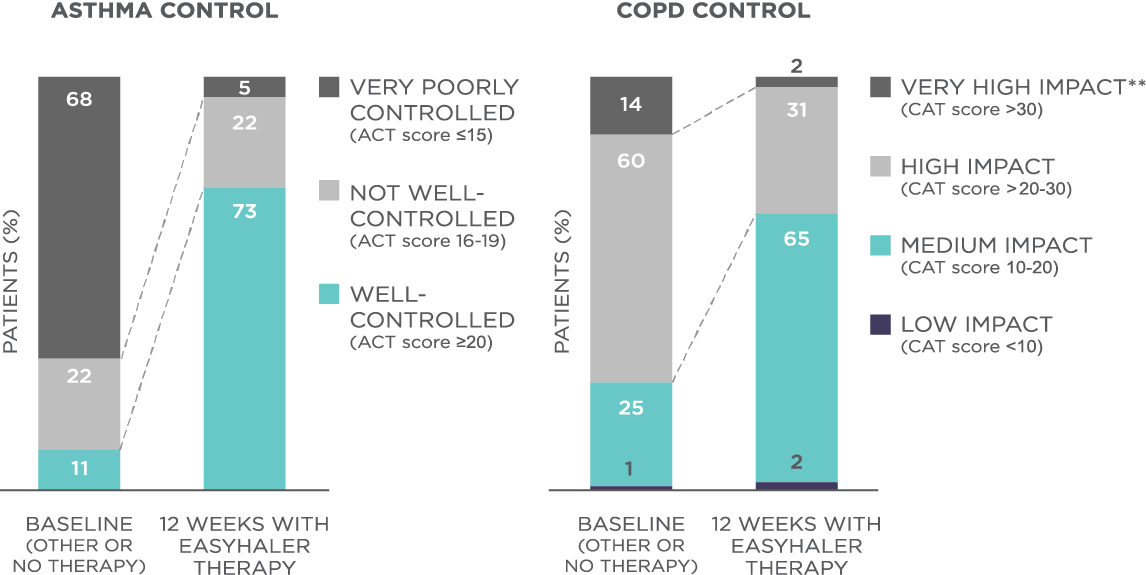
Figure 1. Fobumix Easyhaler® therapy improves disease control among patients with asthma, COPD, and ACO. N(total)=1498 patients (621 with asthma, 778 with COPD, and 99 with ACO; 455 newly diagnosed patients and 1043 patients switching from another inhaler device). **Impact of COPD symptoms on everyday life. Patients received 12 weeks of budesonide/formoterol Fobumix Easyhaler® therapy.
This real-life study showed that Fobumix Easyhaler® significantly improved disease control, quality of life and lung function among patients with asthma, COPD, and ACO when patients were switched from MDI, Turbohaler® and Accuhaler®. The inhaler was considered easy to teach and use, and the majority of patients rated the user satisfaction of Fobumix Easyhaler® with the highest grade on the scale used.
Fobumix Easyhaler® Adverse Events: Common; Candida infections in the oropharynx, Headache, tremor, palpitations, Mild irritation in the throat, coughing, dysphonia including hoarseness. 160/4.5mcg & 320/9mcg strengths: pneumonia (in COPD patients).
Prescribers should consult the SmPC in relation to other side effects.
Fobumix Easyhaler® is indicated for the regular treatment of asthma where use of a combination (inhaled corticosteroid and long-acting β2-adrenoceptor agonist) is appropriate: patients not adequately controlled with inhaled corticosteroids and “as needed” inhaled short-acting β2-adrenoceptor agonists or patients already adequately controlled on both inhaled corticosteroids and long-acting β2-adrenoceptor agonists. Fobumix Easyhaler® 160/4.5 and 320/9 is indicated for symptomatic treatment of adults (18 years and older) with COPD with FEV1 < 70% predicted normal (post- bronchodilator) and an exacerbation history despite regular bronchodilator therapy.
*This article refers to UK brand names. Within the clinical papers the brand names reflect the location of where the studies took place.
References:
- Tamasi L et.al. Adv Ther 2018;35(8):1140-52
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Orion Pharma (UK) Ltd on 01635 520300.
September 2025 / RESP-330bdb(3)

